Testosterone deficiency worsens mitochondrial dysfunction in APP/PS1 mice
- PMID: 38752208
- PMCID: PMC11094339
- DOI: 10.3389/fnagi.2024.1390915
Testosterone deficiency worsens mitochondrial dysfunction in APP/PS1 mice
Abstract
Background: Recent studies show testosterone (T) deficiency worsens cognitive impairment in Alzheimer's disease (AD) patients. Mitochondrial dysfunction, as an early event of AD, is becoming critical hallmark of AD pathogenesis. However, currently, whether T deficiency exacerbates mitochondrial dysfunction of men with AD remains unclear.
Objective: The purpose of this study is to explore the effects of T deficiency on mitochondrial dysfunction of male AD mouse models and its potential mechanisms.
Methods: Alzheimer's disease animal model with T deficiency was performed by castration to 3-month-old male APP/PS1 mice. Hippocampal mitochondrial function of mice was analyzed by spectrophotometry and flow cytometry. The gene expression levels related to mitochondrial biogenesis and mitochondrial dynamics were determined through quantitative real-time PCR (qPCR) and western blot analysis. SH-SY5Y cells treated with flutamide, T and/or H2O2 were processed for analyzing the potential mechanisms of T on mitochondrial dysfunction.
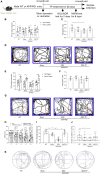
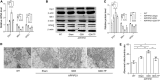
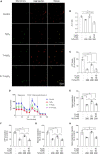
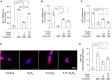
Results: Testosterone deficiency significantly aggravated the cognitive deficits and hippocampal pathologic damage of male APP/PS1 mice. These effects were consistent with exacerbated mitochondrial dysfunction by gonadectomy to male APP/PS1 mice, reflected by further increase in oxidative damage and decrease in mitochondrial membrane potential, complex IV activity and ATP levels. More importantly, T deficiency induced the exacerbation of compromised mitochondrial homeostasis in male APP/PS1 mice by exerting detrimental effects on mitochondrial biogenesis and mitochondrial dynamics at mRNA and protein level, leading to more defective mitochondria accumulated in the hippocampus. In vitro studies using SH-SY5Y cells validated T's protective effects on the H2O2-induced mitochondrial dysfunction, mitochondrial biogenesis impairment, and mitochondrial dynamics imbalance. Administering androgen receptor (AR) antagonist flutamide weakened the beneficial effects of T pretreatment on H2O2-treated SH-SY5Y cells, demonstrating a critical role of classical AR pathway in maintaining mitochondrial function.
Conclusion: Testosterone deficiency exacerbates hippocampal mitochondrial dysfunction of male APP/PS1 mice by accumulating more defective mitochondria. Thus, appropriate T levels in the early stage of AD might be beneficial in delaying AD pathology by improving mitochondrial biogenesis and mitochondrial dynamics.
Keywords: Alzheimer’s disease; hippocampus; mitochondrial biogenesis; mitochondrial dynamics; mitochondrial dysfunction; testosterone deficiency.
Copyright © 2024 Zhang, Chu, Wang, Wang, Wang, Ji, Zhang, Shi, Cui and Kang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Danggui Buxue decoction ameliorates mitochondrial biogenesis and cognitive deficits through upregulating histone H4 lysine 12 acetylation in APP/PS1 mice.J Ethnopharmacol. 2023 Sep 15;313:116554. doi: 10.1016/j.jep.2023.116554. Epub 2023 May 1. J Ethnopharmacol. 2023. PMID: 37137453
-
Mitochondrial damage-induced abnormal glucose metabolism with ageing in the hippocampus of APP/PS1 mice.Metabolomics. 2023 Jun 8;19(6):56. doi: 10.1007/s11306-023-02023-9. Metabolomics. 2023. PMID: 37289288
-
Vascular endothelial growth factor alleviates mitochondrial dysfunction and suppression of mitochondrial biogenesis in models of Alzheimer's disease.Int J Neurosci. 2021 Feb;131(2):154-162. doi: 10.1080/00207454.2020.1733564. Epub 2020 Mar 5. Int J Neurosci. 2021. PMID: 32083964
-
Rhein Ameliorates Cognitive Impairment in an APP/PS1 Transgenic Mouse Model of Alzheimer's Disease by Relieving Oxidative Stress through Activating the SIRT1/PGC-1α Pathway.Oxid Med Cell Longev. 2022 Mar 22;2022:2524832. doi: 10.1155/2022/2524832. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35360200 Free PMC article.
-
Mitochondrial dysfunction and Alzheimer's disease: pathogenesis of mitochondrial transfer.Front Aging Neurosci. 2024 Dec 17;16:1517965. doi: 10.3389/fnagi.2024.1517965. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39741520 Free PMC article. Review.
Cited by
-
Bridging systemic metabolic dysfunction and Alzheimer's disease: the liver interface.Mol Neurodegener. 2025 May 28;20(1):61. doi: 10.1186/s13024-025-00849-6. Mol Neurodegener. 2025. PMID: 40437610 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

