Impact of Intrapartum Azithromycin on the Carriage and Antibiotic Resistance of Escherichia coli and Klebsiella pneumoniae in Mothers and Their Newborns: A Substudy of a Randomized, Double-Blind Trial Conducted in The Gambia and Burkina Faso
- PMID: 38752311
- PMCID: PMC11650870
- DOI: 10.1093/cid/ciae280
Impact of Intrapartum Azithromycin on the Carriage and Antibiotic Resistance of Escherichia coli and Klebsiella pneumoniae in Mothers and Their Newborns: A Substudy of a Randomized, Double-Blind Trial Conducted in The Gambia and Burkina Faso
Abstract
Background: Limited data exist on the effects of intrapartum azithromycin on the prevalence of carriage and antibiotic resistance of Enterobacterales.
Methods: We conducted a randomized trial in The Gambia and Burkina Faso where women received intrapartum azithromycin (2 g) or placebo. We determined the impact of treatment on the prevalence of carriage and antibiotic resistance of Escherichia coli and Klebsiella pneumoniae by analyzing rectal swabs (RS), nasopharyngeal swabs (NPS), breast milk, and rectovaginal swabs (RVS). Bacteria were isolated microbiologically; antibiotic susceptibility was confirmed with an E-test. Prevalence ratios (PRs) with 95% confidence intervals (CIs) were used for comparison between arms.
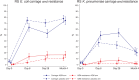
Results: In infants, E. coli carriage in RS was lower in the intervention than in the placebo arm at day 6 (63.0% vs 75.2%; PR, 0.84; 95% CI, .75-.95) and day 28 (52.7% vs 70.4%; 0.75; 0.64-0.87) post-intervention. Prevalence of azithromycin-resistant E. coli was higher in the azithromycin arm at day 6 (13.4% vs 3.6%; 3.75; 1.83-7.69) and day 28 (16.4% vs 9.6%; 1.71; 1.05-2.79). For K. pneumoniae, carriage in RS was higher in the intervention than in the placebo arm at day 6 (49.6% vs 37.2%, 1.33; 1.08-1.64) and day 28 (53.6% vs 32.9%, 1.63; 1.31-2.03). Prevalence of azithromycin-resistant K. pneumoniae was higher in the azithromycin arm at day 28 (7.3% vs 2.1%; 3.49; 1.30-9.37). No differences were observed for other sample types.
Conclusions: Intrapartum azithromycin decreased E. coli carriage but increased both K. pneumoniae carriage and azithromycin resistance in both bacteria. These data need to be considered together with efficacy results to balance the potential short- and long-term impact of the intervention. Clinical Trials Registration. www.clinicaltrials.gov: NCT03199547.
Keywords: Escherichia coli; Klebsiella pneumoniae; antibiotic resistance; bacterial carriage; intrapartum azithromycin.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


References
-
- Okomo U, Akpalu ENK, Le Doare K, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis 2019; 19:1219–1234. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

