Effects of a respiratory and neurological rehabilitation treatment plan in post Covid-19 affected university students. Randomized clinical study
- PMID: 38752418
- PMCID: PMC11100389
- DOI: 10.1177/14799731241255967
Effects of a respiratory and neurological rehabilitation treatment plan in post Covid-19 affected university students. Randomized clinical study
Abstract
Background: COVID-19 demonstrated the possibility of neurological complications such as loss of sense of smell and taste, together with respiratory problems. Respiratory training and rehabilitation of neurological sequelae are essential to improve respiratory function and thus quality of life, and the aim of this study is to evaluate the efficacy of a pulmonary and neurological rehabilitation program.
Objectives: To apply a treatment to reduce dyspnea, increase exertional capacity, increase vital capacity and respiratory muscle strength, together with an increase in olfactory and gustatory sensitivity in post-SARS-CoV-2 patients.
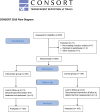
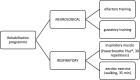
Methods: A randomised controlled experimental study was conducted in 220 patients with a medical diagnosis of COVID-19 and more than 5 months of evolution, dyspnoea or perceived fatigue, including olfactory and gustatory perception problems, of whom 200 patients completed the study. 100 patients were randomly assigned to the intervention group, consisting of an inspiratory training treatment plan (Powerbreathe Plus®) combined with aerobic exercise and olfactory gustatory treatment for 31 days, and 100 patients to the control group, for 31 days without any type of therapy.
Results: The study was conducted in post-Covid-19 patients for 5 months. Two hundred patients were divided into an intervention group (n = 100) and a control group (n = 100). The comparison between the groups showed significant differences in spirometric variables; forced vital capacity (p < .001; Eta2 (0.439); Mean: 0,6135), the ratio between both FEV1/FVC (p < 0.01; Eta2 (0.728); Mean:9,313), peak inspiratory pressure (p < 0.01; Eta2 (0.906); Mean:4,526); changes were observed in dyspnoea measured with the modified Borg scale (p < 0.01; Eta2 (0.811); Mean:1,481) and the modified Medical Research Council scale (p < 0.01; Eta2 (0.881); Mean: 0.777); finally, changes were found in neurological variables, in the questions of the Singapore Smell and Taste Questionnaire, How was your sense of smell after treatment? (p < 0.01; Eta2 (0.813); Mean: 1,721) and How is your sense of taste after treatment? (p < 0.01; Eta2 (0.898); Mean: 1,088).
Conclusion: The implementation of a respiratory rehabilitation treatment plan with the Powerbreathe Plus® device, aerobic exercise and neurorehabilitation with olfactory and gustatory training, is a therapeutic option against respiratory and neurological sequelae in patients who have suffered such sequelae due to the SARS-CoV-2 virus. Clinicaltrials.gov: NCT05195099. First posted 18/01/2022; Last Update Posted 29/06/2022.
Keywords: Covid-19; dysphonea; neurorehabilitation; physiotherapy; respiratory therapy.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Effect of respiratory rehabilitation on quality of life in individuals with post-COVID-19 symptoms: A randomised controlled trial.Ann Phys Rehabil Med. 2025 Feb;68(1):101920. doi: 10.1016/j.rehab.2024.101920. Epub 2025 Jan 11. Ann Phys Rehabil Med. 2025. PMID: 39798250 Clinical Trial.
-
Long COVID-19: Impact of a personalized rehabilitation program.Rehabilitacion (Madr). 2025 Apr-Jun;59(2):100903. doi: 10.1016/j.rh.2025.100903. Epub 2025 Mar 28. Rehabilitacion (Madr). 2025. PMID: 40156968
-
Efficacy of home-based inspiratory muscle training in patients post-covid-19: Protocol for a randomized clinical trial.PLoS One. 2023 May 4;18(5):e0279310. doi: 10.1371/journal.pone.0279310. eCollection 2023. PLoS One. 2023. PMID: 37141260 Free PMC article.
-
Respiratory muscle training in children and adults with neuromuscular disease.Cochrane Database Syst Rev. 2019 Sep 5;9(9):CD011711. doi: 10.1002/14651858.CD011711.pub2. Cochrane Database Syst Rev. 2019. PMID: 31487757 Free PMC article.
-
Inspiratory muscle training improves respiratory muscle strength, functional capacity and quality of life in patients with chronic kidney disease: a systematic review.J Physiother. 2017 Apr;63(2):76-83. doi: 10.1016/j.jphys.2017.02.016. Epub 2017 Mar 14. J Physiother. 2017. PMID: 28433237
Cited by
-
Effects of therapeutic interventions on long COVID: a meta-analysis of randomized controlled trials.EClinicalMedicine. 2025 Aug 5;87:103412. doi: 10.1016/j.eclinm.2025.103412. eCollection 2025 Sep. EClinicalMedicine. 2025. PMID: 40808967 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous