Toripalimab plus chemotherapy in American patients with recurrent or metastatic nasopharyngeal carcinoma: A cost-effectiveness analysis
- PMID: 38752448
- PMCID: PMC11097128
- DOI: 10.1002/cam4.7243
Toripalimab plus chemotherapy in American patients with recurrent or metastatic nasopharyngeal carcinoma: A cost-effectiveness analysis
Abstract
Background: Toripalimab, combined with gemcitabine and cisplatin, has been approved as the first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (RM-NPC), representing a significant milestone as the first FDA-approved innovative therapy for this condition. Despite this achievement, there's a lack of data on the cost-effectiveness of toripalimab for RM-NPC patients in the American context.
Methods: To assess the cost-effectiveness of toripalimab plus chemotherapy versus chemotherapy alone, a 3-state partitioned survival model was constructed. The study involved participants with characteristics matching those in the JUPITER-02 trial. Cost and utility inputs were collected from literature. Main outcomes measured were quality-adjusted life year (QALY), and incremental cost-effectiveness ratio (ICER). Univariate and probabilistic sensitivity analyses, subgroup analyses, and scenario analyses were conducted to verify the robustness of results.
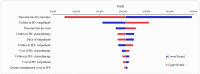
Results: The study found that the toripalimab regimen resulted in 4.390 QALYs at a cost of $361,813, while the chemotherapy-only regimen yielded 1.685 QALYs at a cost of $161,632. This translates to an ICER of $74,004/QALY, below the willingness-to-pay threshold of $150,000/QALY. Sensitivity analyses indicated that utility values, discount rate, and the price of toripalimab significantly impact INMB. With an 87.10% probability of being cost-effective at a $150,000/QALY threshold, the probabilistic sensitivity analysis supports toripalimab plus chemotherapy as a viable option. Scenario analysis showed that toripalimab remains cost-effective unless its price increases by 125%. Additionally, a simulated 15-year study period increases the ICER to $88,026/QALY. Subgroup analysis revealed ICERs of $76,538/QALY for PD-L1 positive and $70,158/QALY for PD-L1 negative groups.
Conclusions: Toripalimab in combination with chemotherapy is likely to be a cost-effective alternative to standard chemotherapy for American patients with RM-NPC. This evidence can guide clinical and reimbursement decision-making in treating RM-NPC patients.
Keywords: cost effectiveness; immunotherapy; nasopharyngeal carcinoma; toripalimab.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors made no disclosures.
Figures

Similar articles
-
Budget impact analysis of the novel PD-1 inhibitor toripalimab versus pembrolizumab in recurrent or metastatic nasopharyngeal carcinoma.J Med Econ. 2024;27(sup3):9-23. doi: 10.1080/13696998.2024.2379055. Epub 2024 Aug 18. J Med Econ. 2024. PMID: 39016811
-
Immune checkpoint inhibition in first-line treatment for recurrent or metastatic nasopharyngeal carcinoma: A CAPTAIN-1st and JUPITER-02 trial-based cost-effectiveness analysis.Oral Oncol. 2022 May;128:105842. doi: 10.1016/j.oraloncology.2022.105842. Epub 2022 Apr 12. Oral Oncol. 2022. PMID: 35428025 Clinical Trial.
-
Toripalimab Plus Chemotherapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: The JUPITER-02 Randomized Clinical Trial.JAMA. 2023 Nov 28;330(20):1961-1970. doi: 10.1001/jama.2023.20181. JAMA. 2023. PMID: 38015220 Free PMC article. Clinical Trial.
-
Cost-effectiveness analysis of gemcitabine plus cisplatin versus fluorouracil plus cisplatin in the first-line setting for Chinese patients with metastatic nasopharyngeal carcinoma.Eur Arch Otorhinolaryngol. 2020 Feb;277(2):577-584. doi: 10.1007/s00405-019-05714-z. Epub 2019 Nov 12. Eur Arch Otorhinolaryngol. 2020. PMID: 31720816 Clinical Trial.
-
Topotecan for the treatment of recurrent and stage IVB carcinoma of the cervix.Health Technol Assess. 2010 May;14 Suppl 1:55-62. doi: 10.3310/hta14Suppl1/08. Health Technol Assess. 2010. PMID: 20507804 Review.
Cited by
-
Leveraging liquid biopsy to uncover resistance mechanisms and guide personalized immunotherapy.Transl Oncol. 2025 Sep;59:102445. doi: 10.1016/j.tranon.2025.102445. Epub 2025 Jun 24. Transl Oncol. 2025. PMID: 40561796 Free PMC article. Review.
References
-
- International Agency for Research on Cancer . Cancer today 2023. Available from: https://gco.iarc.fr/today/home
-
- Zhang M‐X, Li J, Shen G‐P, et al. Intensity‐modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two‐dimensional radiotherapy: a 10‐year experience with a large cohort and long follow‐up. Eur J Cancer. 2015;51(17):2587‐2595. doi:10.1016/j.ejca.2015.08.006 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

