Oral preexposure prophylaxis uptake, adherence, and persistence during periconception periods among women in South Africa
- PMID: 38752557
- PMCID: PMC11211057
- DOI: 10.1097/QAD.0000000000003925
Oral preexposure prophylaxis uptake, adherence, and persistence during periconception periods among women in South Africa
Abstract
Objective: We developed the Healthy Families-PrEP intervention to support HIV-prevention during periconception and pregnancy. We evaluated preexposure prophylaxis (PrEP) use with three objective measures.
Design: This single-arm intervention study enrolled women in KwaZulu-Natal, South Africa, who were HIV-uninfected, not pregnant, in a relationship with a partner with HIV or unknown-serostatus, and with pregnancy plans. PrEP was offered as part of a comprehensive HIV prevention intervention. Participants were followed for 12 months.
Methods: We evaluated periconception PrEP uptake and adherence using quarterly plasma tenofovir concentrations. We modeled factors associated with PrEP uptake and high plasma tenofovir (past day dosing). Patterns of use were analyzed using electronic pillcap data. Dried blood spots to measure intracellular tenofovir product (past 2 months dosing) were analyzed for a subset of women.
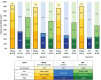
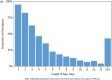
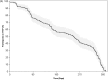
Results: Three hundred thirty women with median age 24 (IQR: 22-27) years enrolled. Partner HIV-serostatus was unknown by 96% ( N = 316); 60% (195) initiated PrEP. High plasma tenofovir concentrations were seen in 35, 25, 22, and 20% of samples at 3, 6, 9, and 12 months, respectively. Similar adherence was measured by pillcap and dried blood spots. In adjusted models, lower income, alcohol use, and higher HIV stigma were associated with high plasma tenofovir. Eleven HIV-seroconversions were observed (incidence rate: 4.04/100 person-years [95% confidence interval: 2.24-7.30]). None had detectable plasma tenofovir.
Conclusion: The Healthy Families-PrEP intervention supported women in PrEP use. We observed high interest in periconception PrEP and over one-third adhered to PrEP in the first quarter; one-fifth were adherent over a year. High HIV incidence highlights the importance of strategies to reduce HIV incidence among periconception women.
Clinical trial number: NCT03194308.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr Matthews received operational support from Gilead Sciences.
Dr Haberer has been a consultant for Merck and owns stock in Natera.
Dr Hurwitz and Ms. Bennett are employed by and own equity in Target RWE, which has received fees from Amgen, Baxter International, Gilead Sciences, Janssen Research & Development (Janssen R&D), and Merck outside the submitted work.
Dr Baeten is an employee of Gilead Sciences, outside of the present work.
Dr Hendrix has received research funding from Gilead Sciences and Merck and is founder and a nonfiduciary manager of Prionde Biopharma, LLC, outside of the present work. There are no conflicts of interest for the remaining authors.
Figures

References
-
- UNAIDS. Country fact sheets: South Africa (2021) 2022 [cited 8 September 2022]. https://www.unaids.org/en/regionscountries/countries/southafrica.
-
- Department of Health, Republic of South Africa. Guidelines for the provision of pre-exposure prophylaxis (PrEP) to persons at substantial risk of HIV infection. 2020.
-
- Medicine Controls Council. Press release: Medicines Control Council approves fixed-dose combination of tenofovir disoproxyl fumarate and emtricitabine for preexposure prophylaxis of HIV 2015 [cited 25 March 2023]. https://www.sahpra.org.za/wp-content/uploads/2020/01/6614b94510.11_Media....
-
- World Health Organization. Technical brief: PREVENTING HIV DURING PREGNANCY AND BREASTFEEDING IN THE CONTEXT OF PREP. Geneva, Switzerland: WHO; 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

