Calcipotriol Nanosuspension-Loaded Trilayer Dissolving Microneedle Patches for the Treatment of Psoriasis: In Vitro Delivery and In Vivo Antipsoriatic Activity Studies
- PMID: 38752564
- PMCID: PMC11151207
- DOI: 10.1021/acs.molpharmaceut.3c01223
Calcipotriol Nanosuspension-Loaded Trilayer Dissolving Microneedle Patches for the Treatment of Psoriasis: In Vitro Delivery and In Vivo Antipsoriatic Activity Studies
Abstract
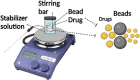
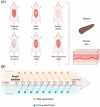
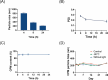
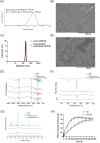
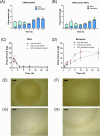
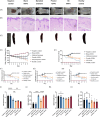
Psoriasis, affecting 2-3% of the global population, is a chronic inflammatory skin condition without a definitive cure. Current treatments focus on managing symptoms. Recognizing the need for innovative drug delivery methods to enhance patient adherence, this study explores a new approach using calcipotriol monohydrate (CPM), a primary topical treatment for psoriasis. Despite its effectiveness, CPM's therapeutic potential is often limited by factors like the greasiness of topical applications, poor skin permeability, low skin retention, and lack of controlled delivery. To overcome these challenges, the study introduces CPM in the form of nanosuspensions (NSs), characterized by an average particle size of 211 ± 2 nm. These CPM NSs are then incorporated into a trilayer dissolving microneedle patch (MAP) made from poly(vinylpyrrolidone) and w poly(vinyl alcohol) as needle arrays and prefrom 3D printed polylactic acid backing layer. This MAP features rapidly dissolving tips and exhibits good mechanical properties and insertion capability with delivery efficiency compared to the conventional Daivonex ointment. The effectiveness of this novel MAP was tested on Sprague-Dawley rats with imiquimod-induced psoriasis, demonstrating efficacy comparable to the marketed ointment. This innovative trilayer dissolving MAP represents a promising new local delivery system for calcipotriol, potentially revolutionizing psoriasis treatment by enhancing drug delivery and patient compliance.
Keywords: calcipotriol; microneedle; nanocrystal; nanosuspension; psoriasis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Nanosuspension-Loaded Dissolving Microneedle Patches for Enhanced Transdermal Delivery of a Highly Lipophilic Cannabidiol.Int J Nanomedicine. 2024 May 7;19:4061-4079. doi: 10.2147/IJN.S452207. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38736651 Free PMC article.
-
Development and comparative analysis of clobetasol-loaded microneedle patches versus clobetasol propionate ointment in experimental induced-psoriasis model.Int J Pharm. 2025 Apr 15;674:125423. doi: 10.1016/j.ijpharm.2025.125423. Epub 2025 Mar 10. Int J Pharm. 2025. PMID: 40074158
-
Dissolving microneedle patch for transdermal delivery of perindopril erbumine.Inflammopharmacology. 2025 Mar;33(3):1381-1391. doi: 10.1007/s10787-025-01696-z. Epub 2025 Feb 26. Inflammopharmacology. 2025. PMID: 40009346
-
Management of psoriasis with calcipotriol used as monotherapy.J Am Acad Dermatol. 1997 Sep;37(3 Pt 2):S53-4. J Am Acad Dermatol. 1997. PMID: 9344185 Review.
-
Calcipotriol ointment. A review of its use in the management of psoriasis.Am J Clin Dermatol. 2001;2(2):95-120. doi: 10.2165/00128071-200102020-00008. Am J Clin Dermatol. 2001. PMID: 11705309 Review.
Cited by
-
Novel Small-Molecule Treatment and Emerging Biological Therapy for Psoriasis.Biomedicines. 2025 Mar 23;13(4):781. doi: 10.3390/biomedicines13040781. Biomedicines. 2025. PMID: 40299379 Free PMC article. Review.
-
Microneedles as an Emerging Platform for Transdermal Delivery of Phytochemicals.Mol Pharm. 2024 Dec 2;21(12):6007-6033. doi: 10.1021/acs.molpharmaceut.4c00894. Epub 2024 Oct 29. Mol Pharm. 2024. PMID: 39470172 Free PMC article. Review.
-
Pathophysiology and Treatment of Psoriasis: From Clinical Practice to Basic Research.Pharmaceutics. 2025 Jan 3;17(1):56. doi: 10.3390/pharmaceutics17010056. Pharmaceutics. 2025. PMID: 39861704 Free PMC article. Review.
-
Bimodal Dissolving Microneedles with Nanoparticle Coating and Encapsulation for Extended Dual-Drug Delivery.Small. 2025 Jul;21(30):e2502904. doi: 10.1002/smll.202502904. Epub 2025 May 26. Small. 2025. PMID: 40417960 Free PMC article.
References
-
- Tekko I. A.; Permana A. D.; Vora L.; Hatahet T.; McCarthy H. O.; Donnelly R. F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 10546910.1016/j.ejps.2020.105469. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

