Anastrozole Dose Escalation for Optimal Estrogen Suppression in Postmenopausal Early-Stage Breast Cancer: A Prospective Trial
- PMID: 38752717
- PMCID: PMC11292197
- DOI: 10.1158/1078-0432.CCR-24-0341
Anastrozole Dose Escalation for Optimal Estrogen Suppression in Postmenopausal Early-Stage Breast Cancer: A Prospective Trial
Abstract
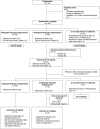
Purpose: We previously reported that postmenopausal women with estrogen receptor-α-positive breast cancer receiving adjuvant anastrozole 1 mg/day (ANA1) with estrone (E1) ≥1.3 pg/mL and estradiol (E2) ≥0.5 pg/mL [inadequate estrogen suppression (IES)] had a threefold increased risk of a breast cancer event. The objective of this study was to determine if increasing anastrozole to 10 mg/day (ANA10) could result in adequate estrogen suppression (AES: E1 <1.3 pg/mL and/or E2 <0.5 pg/mL) among those with IES on ANA1.
Patients and methods: Postmenopausal women with estrogen receptor-α-positive breast cancer planning to receive adjuvant ANA1 were eligible. E1 and E2 were assessed pre- and post-8 to 10 weeks of ANA1. Those with IES were switched to 8- to 10-week cycles of ANA10 followed by letrozole 2.5 mg/day. E1 and E2 were assessed after each cycle. Anastrozole concentrations were measured post-ANA1 and post-ANA10. Primary analyses included patients who documented taking at least 80% of the planned treatment (adherent cohort).
Results: In total, 132 (84.6%) of 156 eligible patients were ANA1 adherent. IES occurred in 40 (30.3%) adherent patients. Twenty-five (78.1%) of 32 patients who began ANA10 were adherent, and AES was achieved in 19 (76.0%; 90% confidence interval, 58.1%-89.0%) patients. Anastrozole concentrations post-ANA1 and post-ANA10 did not differ by estrogen suppression status among adherent patients. AES was maintained/attained in 21 (91.3%) of 23 letrozole-adherent patients.
Conclusions: Approximately 30% of ANA1-adherent patients had IES. Among those who switched to ANA10 and were adherent, 76% had AES. Further studies are required to validate emerging data that ANA1 results in IES for some patients and to determine the clinical benefit of switching to ANA10 or an alternative aromatase inhibitor.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
T.C. Haddad reports grants from NCI during the conduct of the study, research funding to the institution from Takeda Oncology, and other support to the institution from Puma Biotechnology outside the submitted work. L. Wang reports grants from NCI during the conduct of the study. S. Chumsri reports research funding to the institution from Merck & Co., Pfizer, Salix Pharmaceuticals, Rebiotix Inc, Novartis, and BriaCell Therapeutics and other support to the institution from AstraZeneca, Daiichi Sankyo, Immunomedics, Biotheranostics, Novartis, Athenex, Syndax, Puma Biotechnology, Eisai, and Seagen Rebiotix Inc. M.P. Goetz reports grants from NCI during the conduct of the study and research funding to Mayo Clinic from Lilly, Pfizer, Sermonix, Loxo, AstraZeneca and ATOSSA Therapeutics; consulting fees to the institution from ARC Therapeutics, AstraZeneca, Biotheranostics, Blueprint Medicines, Lilly, Novartis, RNA Diagnostics, Sanofi Genzyme, Seattle Genetics, Sermonix, Engage Health Media, Laekna and TerSera Therapeutics; and personal fees (CME) from Research to Practice, Clinical Education Alliance, Medscape, MJH Life Sciences, Total Health Conferencing, and Curio Science. C.C. O’Sullivan reports research funding to the institution from Genentech, Bavarian Nordic, Sermonix, Seagen, Tesaro and Nference, and other support to the institution from AstraZeneca Enhertu Steering Committee, Medscape Presentation, Seagen HER2CLIMB-05 Clinical Study Steering Committee, Edith Sanford Presentation, Seagen Breast Cancer Advisory Board, AstraZeneca Dato-DXd Advisory Board, and AstraZeneca HER2 Steering Commitee. K.V. Giridhar reports other support to the institution from Lilly, Novartis, AstraZeneca, and Puma Biotechnology.
Figures

References
-
- Ingle JN. Overview of adjuvant trials of aromatase inhibitors in early breast cancer. Steroids 2011;76:765–7. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52. - PubMed
-
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med 2003;348:2431–42. - PubMed
-
- Ryan KJ. Biological aromatization of steroids. J Biol Chem 1959;234:268–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

