Long-term hematopoietic transfer of the anti-cancer and lifespan-extending capabilities of a genetically engineered blood system by transplantation of bone marrow mononuclear cells
- PMID: 38752723
- PMCID: PMC11098557
- DOI: 10.7554/eLife.88275
Long-term hematopoietic transfer of the anti-cancer and lifespan-extending capabilities of a genetically engineered blood system by transplantation of bone marrow mononuclear cells
Abstract
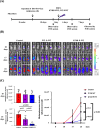
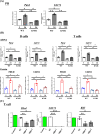
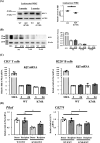
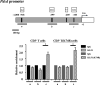
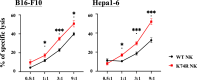
A causal relationship exists among the aging process, organ decay and disfunction, and the occurrence of various diseases including cancer. A genetically engineered mouse model, termed Klf1K74R/K74R or Klf1(K74R), carrying mutation on the well-conserved sumoylation site of the hematopoietic transcription factor KLF1/EKLF has been generated that possesses extended lifespan and healthy characteristics, including cancer resistance. We show that the healthy longevity characteristics of the Klf1(K74R) mice, as exemplified by their higher anti-cancer capability, are likely gender-, age-, and genetic background-independent. Significantly, the anti-cancer capability, in particular that against melanoma as well as hepatocellular carcinoma, and lifespan-extending property of Klf1(K74R) mice, could be transferred to wild-type mice via transplantation of their bone marrow mononuclear cells at a young age of the latter. Furthermore, NK(K74R) cells carry higher in vitro cancer cell-killing ability than wild-type NK cells. Targeted/global gene expression profiling analysis has identified changes in the expression of specific proteins, including the immune checkpoint factors PDCD and CD274, and cellular pathways in the leukocytes of the Klf1(K74R) that are in the directions of anti-cancer and/or anti-aging. This study demonstrates the feasibility of developing a transferable hematopoietic/blood system for long-term anti-cancer and, potentially, for anti-aging.
Keywords: EKLF/ KLF1; aging; anti-cancer; bone marrow transplantation; leukocytes; mouse; regenerative medicine; stem cells.
© 2023, Wang, Hung et al.
Conflict of interest statement
JW, CH, YL, CL, KY, KW, ZL, BC, TH, YS, PH, LC, TC, CY, NL, CS No competing interests declared, TL is an employee of Pro-Clintech Co. Ltd
Figures


Update of
- doi: 10.1101/2023.04.21.537849
- doi: 10.7554/eLife.88275.1
- doi: 10.7554/eLife.88275.2
References
-
- Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen T, Tavernarakis N, Rubinsztein DC, Partridge L, Kroemer G, Labbadia J, Fang EF. Autophagy in healthy aging and disease. Nature Aging. 2021;1:634–650. doi: 10.1038/s43587-021-00098-4. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

