Breakthrough Invasive Fungal Infections in Patients With High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review
- PMID: 38752732
- PMCID: PMC11259221
- DOI: 10.1093/cid/ciae203
Breakthrough Invasive Fungal Infections in Patients With High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review
Abstract
Background: Primary antifungal prophylaxis with mold-active azoles is used to prevent invasive fungal infections in patients with high-risk hematological disorders; however, breakthrough infections occur, and the reasons for treatment failure are still not fully understood. To help inform clinical decisions, we sought to define microbiological, clinical, and pharmacological characteristics of proven and probable breakthrough invasive fungal infections (bIFIs) in patients with high-risk hematological disorders receiving voriconazole or posaconazole prophylaxis.
Methods: We performed a systematic review of the literature following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The search strategy was last conducted on 19 April 2023.
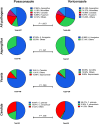
Results: We assessed 5293 studies for eligibility, and 300 were selected for data extraction. These studies described 1076 cases of bIFIs occurring under voriconazole (42.5%) or posaconazole (57.5%). The most commonly found pathogens were Aspergillus (40%), Mucorales (20%), Candida (18%), and Fusarium (9%) species. Mucorales were more frequent among voriconazole-emerging cases, whereas Aspergillus and Fusarium were more prevalent among posaconazole-emerging cases. Definitive, putative, or probable antifungal resistance was found in 31% of cases. Therapeutic drug monitoring showed subtherapeutic azole concentration in 32 of 90 (36%) cases. Infection-related mortality was reported in 117 cases and reached 35%.
Conclusions: In our systemic review, the most common bIFIs were aspergillosis, mucormycosis, candidiasis, and fusariosis. Antifungal resistance explains only a minority of cases. Subtherapeutic prophylaxis was frequent but rarely reported. Prospective studies are needed to better understand these infections and to establish optimal management.
Keywords: breakthrough; invasive fungal infections; posaconazole; prophylaxis; voriconazole.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . S. F. D. has received honoraria for consultancy and research funding from AVIR Pharma Inc and Merck & Co. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 2010; 50:1091–100. - PubMed
-
- Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 2012; 73:293–300. - PubMed
-
- Baden LR, Swaminathan S, Almyroudis NG, et al. NCCN guidelines version 2.2023 prevention and treatment of cancer-related infections. National Comprehensive Cancer Network, Inc, 2023. Available at: NCCN.org. Accessed 13 February 2024.
-
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018; 24:e1–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

