Increased frequencies of highly activated regulatory T cells skewed to a T helper 1-like phenotype with reduced suppressive capacity in dengue patients
- PMID: 38752787
- PMCID: PMC11237415
- DOI: 10.1128/mbio.00063-24
Increased frequencies of highly activated regulatory T cells skewed to a T helper 1-like phenotype with reduced suppressive capacity in dengue patients
Abstract
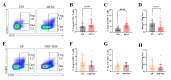
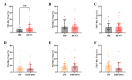
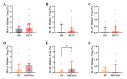
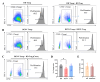
The pathogenesis of dengue involves a complex interplay between the viral factor and the host immune response. A mismatch between the infecting serotype and the adaptive memory response is hypothesized to lead to exacerbated immune responses resulting in severe dengue. Here, we aim to define in detail the phenotype and function of different regulatory T cell (Treg) subsets and their association with disease severity in a cohort of acute dengue virus (DENV)-infected Cambodian children. Treg frequencies and proliferation of Tregs are increased in dengue patients compared to age-matched controls. Tregs from dengue patients are skewed to a Th1-type Treg phenotype. Interestingly, Tregs from severe dengue patients produce more interleukin-10 after in vitro stimulation compared to Tregs from classical dengue fever patients. Functionally, Tregs from dengue patients have reduced suppressive capacity, irrespective of disease severity. Taken together, these data suggest that even though Treg frequencies are increased in the blood of acute DENV-infected patients, Tregs fail to resolve inflammation and thereby could contribute to the immunopathology of dengue.
Importance: According to the World Health Organization, dengue is the fastest-spreading, epidemic-prone infectious disease. The extent of dengue virus infections increased over the years, mainly driven by globalization-including travel and trade-and environmental changes. Dengue is an immunopathology caused by an imbalanced immune response to a secondary heterotypic infection. As regulatory T cells (Tregs) are essential in maintaining immune homeostasis and dampening excessive immune activation, this study addressed the role of Tregs in dengue immunopathology. We show that Tregs from dengue patients are highly activated, skewed to a Th1-like Treg phenotype and less suppressive compared to healthy donor Tregs. Our data suggest that Tregs fail to resolve ongoing inflammation during dengue infection and hence contribute to the immunopathology of severe dengue disease. These data clarify the role of Tregs in dengue immunopathogenesis, emphasizing the need to develop T cell-based vaccines for dengue.
Keywords: FOXP3; dengue virus; regulatory T cells; severe dengue disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Regulatory T-cells in acute dengue viral infection.Immunology. 2018 May;154(1):89-97. doi: 10.1111/imm.12863. Epub 2017 Dec 18. Immunology. 2018. PMID: 29140541 Free PMC article.
-
Altered profile of regulatory T cells and associated cytokines in mild and moderate dengue.Eur J Clin Microbiol Infect Dis. 2016 Mar;35(3):453-61. doi: 10.1007/s10096-015-2561-0. Epub 2016 Feb 9. Eur J Clin Microbiol Infect Dis. 2016. PMID: 26861813
-
B Cell Responses during Secondary Dengue Virus Infection Are Dominated by Highly Cross-Reactive, Memory-Derived Plasmablasts.J Virol. 2016 May 27;90(12):5574-85. doi: 10.1128/JVI.03203-15. Print 2016 Jun 15. J Virol. 2016. PMID: 27030262 Free PMC article.
-
Immunity and immunopathology in dengue virus infections.Semin Immunol. 1992 Apr;4(2):121-7. Semin Immunol. 1992. PMID: 1617166 Review.
-
Meta-Analysis of Alterations in Regulatory T Cells' Frequency and Suppressive Capacity in Patients with Vitiligo.J Immunol Res. 2022 Sep 16;2022:6952299. doi: 10.1155/2022/6952299. eCollection 2022. J Immunol Res. 2022. PMID: 36164321 Free PMC article. Review.
Cited by
-
Toward a deeper understanding of dengue: novel method for quantification and isolation of envelope protein epitope-specific antibodies.mSphere. 2025 May 27;10(5):e0096124. doi: 10.1128/msphere.00961-24. Epub 2025 Apr 11. mSphere. 2025. PMID: 40214258 Free PMC article.
-
The Impact of Vitamin D in the Prevention of Influenza, COVID-19, and Dengue: A Review.Biomedicines. 2025 Apr 9;13(4):927. doi: 10.3390/biomedicines13040927. Biomedicines. 2025. PMID: 40299497 Free PMC article. Review.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060 - DOI - PMC - PubMed
-
- World Health Organization . 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. World Health Organization, Geneva.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

