Cellular resolution contributions to ictal population signals
- PMID: 38752861
- PMCID: PMC11251866
- DOI: 10.1111/epi.17983
Cellular resolution contributions to ictal population signals
Abstract
Objective: The increased amplitude of ictal activity is a common feature of epileptic seizures, but the determinants of this amplitude have not been identified. Clinically, ictal amplitudes are measured electrographically (using, e.g., electroencephalography, electrocorticography, and depth electrodes), but these methods do not enable the assessment of the activity of individual neurons. Population signal may increase from three potential sources: (1) increased synchrony (i.e., more coactive neurons); (2) altered active state, from bursts of action potentials and/or paroxysmal depolarizing shifts in membrane potential; and (3) altered subthreshold state, which includes all lower levels of activity. Here, we quantify the fraction of ictal signal from each source.
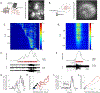
Methods: To identify the cellular determinants of the ictal signal, we measured single cell and population electrical activity and neuronal calcium levels via optical imaging of the genetically encoded calcium indicator (GECI) GCaMP. Spontaneous seizure activity was assessed with microendoscopy in an APP/PS1 mouse with focal cortical injury and via widefield imaging in the organotypic hippocampal slice cultures (OHSCs) model of posttraumatic epilepsy. Single cell calcium signals were linked to a range of electrical activities by performing simultaneous GECI-based calcium imaging and whole-cell patch-clamp recordings in spontaneously seizing OHSCs. Neuronal resolution calcium imaging of spontaneous seizures was then used to quantify the cellular contributions to population-level ictal signal.
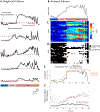
Results: The seizure onset signal was primarily driven by increased subthreshold activity, consistent with either barrages of excitatory postsynaptic potentials or sustained membrane depolarization. Unsurprisingly, more neurons entered the active state as seizure activity progressed. However, the increasing fraction of active cells was primarily driven by synchronous reactivation and not from continued recruitment of new populations of neurons into the seizure.
Significance: This work provides a critical link between single neuron activity and population measures of seizure activity.
Keywords: calcium imaging; epilepsy; recruitment; seizure onset; subthreshold.
© 2024 International League Against Epilepsy.
Conflict of interest statement
Conflict of interest disclosure
None of the authors has any conflict of interest to disclose.
Figures
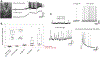



References
-
- Panzica F, Franceschetti S, Binelli S, Canafoglia L, Granata T, Avanzini G, Spectral properties of EEG fast activity ictal discharges associated with infantile spasms. Clin Neurophysiol 110, 593–603 (1999). - PubMed
-
- Engel J Jr., A practical guide for routine EEG studies in epilepsy. J Clin Neurophysiol 1, 109–142 (1984). - PubMed
-
- Noachtar S, Remi J, The role of EEG in epilepsy: a critical review. Epilepsy & behavior : E&B 15, 22–33 (2009). - PubMed
-
- Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydin C, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Hausser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun WL, Svoboda K, Carandini M, Harris KD, Koch C, O'Keefe J, Harris TD, Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

