Osteochondroprogenitor cells and neutrophils expressing p21 and senescence markers modulate fracture repair
- PMID: 38753433
- PMCID: PMC11178538
- DOI: 10.1172/JCI179834
Osteochondroprogenitor cells and neutrophils expressing p21 and senescence markers modulate fracture repair
Abstract
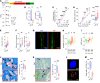
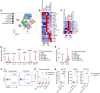
Cells expressing features of senescence, including upregulation of p21 and p16, appear transiently following tissue injury, yet the properties of these cells or how they contrast with age-induced senescent cells remains unclear. Here, we used skeletal injury as a model and identified the rapid appearance following fracture of p21+ cells expressing senescence markers, mainly as osteochondroprogenitors (OCHs) and neutrophils. Targeted genetic clearance of p21+ cells suppressed senescence-associated signatures within the fracture callus and accelerated fracture healing. By contrast, p21+ cell clearance did not alter bone loss due to aging; conversely, p16+ cell clearance, known to alleviate skeletal aging, did not affect fracture healing. Following fracture, p21+ neutrophils were enriched in signaling pathways known to induce paracrine stromal senescence, while p21+ OCHs were highly enriched in senescence-associated secretory phenotype factors known to impair bone formation. Further analysis revealed an injury-specific stem cell-like OCH subset that was p21+ and highly inflammatory, with a similar inflammatory mesenchymal population (fibro-adipogenic progenitors) evident following muscle injury. Thus, intercommunicating senescent-like neutrophils and mesenchymal progenitor cells were key regulators of tissue repair in bone and potentially across tissues. Moreover, our findings established contextual roles of p21+ versus p16+ senescent/senescent-like cells that may be leveraged for therapeutic opportunities.
Keywords: Aging; Bone biology; Bone disease; Cellular senescence.
Figures





Update of
-
Osteochondroprogenitor cells and neutrophils expressing p21 and senescence markers modulate fracture repair.bioRxiv [Preprint]. 2024 Feb 7:2024.02.01.578420. doi: 10.1101/2024.02.01.578420. bioRxiv. 2024. Update in: J Clin Invest. 2024 May 16;134(12):e179834. doi: 10.1172/JCI179834. PMID: 38370844 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

