Preclinical assessment of a recombinant RBD-Fc fusion protein as SARS-CoV-2 candidate vaccine
- PMID: 38753442
- PMCID: PMC11393645
- DOI: 10.1556/1886.2024.00045
Preclinical assessment of a recombinant RBD-Fc fusion protein as SARS-CoV-2 candidate vaccine
Abstract
Background: Waning immunity and emergence of new variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), highlight the need for further research in vaccine development.
Methods: A recombinant fusion protein containing the receptor-binding domain (RBD) fused to the human IgG1 Fc (RBD-Fc) was produced in CHO-K1 cells. RBD-Fc was emulsified with four adjuvants to evaluate its immunogenicity. The RBD-specific humoral and cellular immune responses were assessed by ELISA. The virus neutralizing potency of the vaccine was investigated using four neutralization methods. Safety was studied in mice and rabbits, and Antibody-Dependent Enhancement (ADE) effects were investigated by flow cytometry.
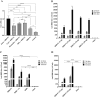
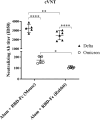
Results: RBD-Fc emulsified in Alum induced a high titer of anti-RBD antibodies with remarkable efficacy in neutralizing both pseudotyped and live SARS-CoV-2 Delta variant. The neutralization potency dropped significantly in response to the Omicron variant. RBD-Fc induced both TH2 and particularly TH1 immune responses. Histopathologic examinations demonstrated no substantial pathologic changes in different organs. No changes in serum biochemical and hematologic parameters were observed. ADE effect was not observed following immunization with RBD-Fc.
Conclusion: RBD-Fc elicits highly robust neutralizing antibodies and cellular immune responses, with no adverse effects. Therefore, it could be considered a promising and safe subunit vaccine against SARS-CoV-2.
Keywords: RBD-Fc; SARS-CoV-2; immunogenicity; neutralization; safety; vaccine.
Conflict of interest statement
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures




Similar articles
-
Receptor binding domain proteins of SARS-CoV-2 variants produced in Nicotiana benthamiana elicit neutralizing antibodies against variants of concern.J Med Virol. 2022 Sep;94(9):4265-4276. doi: 10.1002/jmv.27881. Epub 2022 Jun 1. J Med Virol. 2022. PMID: 35615895 Free PMC article.
-
CHO-produced RBD-Fc subunit vaccines with alternative adjuvants generate immune responses against SARS-CoV-2.PLoS One. 2023 Jul 14;18(7):e0288486. doi: 10.1371/journal.pone.0288486. eCollection 2023. PLoS One. 2023. PMID: 37450510 Free PMC article.
-
Plant-Produced Receptor-Binding Domain of SARS-CoV-2 Elicits Potent Neutralizing Responses in Mice and Non-human Primates.Front Plant Sci. 2021 May 13;12:682953. doi: 10.3389/fpls.2021.682953. eCollection 2021. Front Plant Sci. 2021. PMID: 34054909 Free PMC article.
-
The Influence of Adjuvant Type on the Immunogenicity of RBD/N Cocktail Antigens as a Vaccine Candidate against SARS-CoV-2 Virus.Microbiol Spectr. 2023 Jun 15;11(3):e0256422. doi: 10.1128/spectrum.02564-22. Epub 2023 May 18. Microbiol Spectr. 2023. PMID: 37199661 Free PMC article.
-
A Recombinant Protein XBB.1.5 RBD/Alum/CpG Vaccine Elicits High Neutralizing Antibody Titers against Omicron Subvariants of SARS-CoV-2.Vaccines (Basel). 2023 Oct 1;11(10):1557. doi: 10.3390/vaccines11101557. Vaccines (Basel). 2023. PMID: 37896960 Free PMC article.
Cited by
-
Mucosal Vaccination Against SARS-CoV-2 Using Human Probiotic Bacillus subtilis Spores as an Adjuvant Induces Potent Systemic and Mucosal Immunity.Vaccines (Basel). 2025 Jul 21;13(7):772. doi: 10.3390/vaccines13070772. Vaccines (Basel). 2025. PMID: 40733749 Free PMC article.
-
Quality by design for transient RBD-Fc fusion protein production in Chinese hamster ovary cells.Biotechnol Rep (Amst). 2025 Feb 9;45:e00882. doi: 10.1016/j.btre.2025.e00882. eCollection 2025 Mar. Biotechnol Rep (Amst). 2025. PMID: 40034964 Free PMC article.
-
Fusion protein-based COVID-19 vaccines exemplified by a chimeric vaccine based on a single fusion protein (W-PreS-O).Front Immunol. 2025 Jan 28;16:1452814. doi: 10.3389/fimmu.2025.1452814. eCollection 2025. Front Immunol. 2025. PMID: 39935478 Free PMC article.
References
-
- World Health Organization . COVID-19 weekly epidemiological update. edition 164. 16 February 2024.
-
- Rubin R. COVID-19 vaccines vs variants—determining how much immunity is enough. Jama. 2021;325(13):1241–3. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
