Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial
- PMID: 38753448
- PMCID: PMC11481169
- DOI: 10.1093/cvr/cvae109
Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial
Abstract
Aims: Data describing the long-term efficacy and tolerability of inclisiran are limited. This was explored in ORION-8, an open-label extension of preceding Phase 2 and Phase 3 placebo-controlled and open-label extension trials.
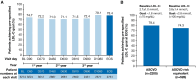
Methods and results: Following completion of the parent trial, adult patients with atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalent, or heterozygous familial hypercholesterolaemia received open-label inclisiran twice yearly (after initial and 3-month doses) until Day 990, followed by an end-of-study visit at Day 1080 or ≥ 90 days after the last dose. The study endpoints included the proportion of patients achieving pre-specified low-density lipoprotein cholesterol (LDL-C) goals [ASCVD: < 1.8 mmol/L (< 70 mg/dL); ASCVD risk equivalent: < 2.6 mmol/L (< 100 mg/dL)], percentage and absolute changes in LDL-C at end-of-study, and safety of inclisiran. Of 3274 patients, 2446 (74.7%) were followed until end-of-study. Mean age was 64.9 ± 9.9 years, 82.7% (n = 2709) had ASCVD, and mean baseline LDL-C was 2.9 ± 1.2 mmol/L. Mean cumulative exposure to inclisiran (including parent trials) was 3.7 years; maximum exposure was 6.8 years. With inclisiran, 78.4% [95% confidence interval (CI): 76.8, 80.0] of patients achieved pre-specified LDL-C goals and mean percentage change in LDL-C was -49.4% (95% CI: -50.4, -48.3). No attenuation of LDL-C lowering over time was observed. Treatment-emergent adverse events at injection site (all mild/moderate) occurred in 5.9% of the patients. Inclisiran-associated anti-drug antibodies were infrequent (5.5%) and had no impact on the efficacy or safety of inclisiran. No new safety signals were identified.
Conclusion: In the largest and longest follow-up to date with >12 000 patient-years exposure, inclisiran demonstrated consistent and effective LDL-C lowering with a favourable long-term safety and tolerability profile.
Trial registration number: ClinicalTrials.gov identifier: NCT03814187.
Keywords: Atherosclerotic cardiovascular disease; Inclisiran; Long-term exposure; Low-density lipoprotein cholesterol; Proprotein convertase subtilisin/kexin type 9.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.S.W. reports receiving advisory board fees from Boehringer Ingelheim and past fees for consulting on lipid issues with The Medicines Company; F.J.R. reports receiving advisory board fees and lecture fees from Amgen, Sanofi-Aventis, Regeneron Pharmaceuticals, Novartis, and LIB Therapeutics; W.K. reports receiving consulting fees and lecture fees from AstraZeneca, Novartis, and Amgen; consulting fees from Pfizer, The Medicines Company, Novartis, DalCor Pharmaceuticals, Kowa, Corvidia Therapeutics, Esperion, Genentech, OMEICOS, Novo Nordisk, LIB Therapeutics, Daiichi Sankyo, New Amsterdam Pharma, and TenSixteen Bio; lecture fees from Berlin-Chemie, Bristol-Myers Squibb, and Sanofi; and grant support and provision of reagents from Singulex, Abbott, Roche Diagnostics, and Dr Beckmann Pharma; U.L. reports receiving grant support from Amgen, Bayer, and Novartis and advisory board fee from Novartis; L.A.L. reports grant support paid to his institution and advisory board fees and fees for CME from Amgen and Novartis; grant support paid to his institution and fees for serving on a steering committee from Kowa, The Medicines Company and Novartis; and advisory board fees and fees for CME from Amarin, AstraZeneca, HLS, Merck, New Amsterdam, Pfizer, and Sanofi; G.G.S. reports receiving research support paid to his institution from AstraZeneca, Sanofi, Silence Therapeutics, and The Medicines Company and a patent (62/806313) on a method for reducing cardiovascular risk assigned in full to the University of Colorado; K.K.R. reports receiving support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre; his institution (Imperial College London) receives support from the NIHR Applied Research Collaboration Northwest London. K.K.R. also reports receiving lecture fees from Aegerion Pharmaceuticals, Kowa, Cipla, Algorithm, and Zuelling Pharma; grant support paid to his institution; lecture fees and advisory board fees from Amgen, Regeneron Pharmaceuticals/Sanofi, and Pfizer; lecture fees and fees for serving on steering committees for trials from AstraZeneca and Eli Lilly; fees for serving on steering committees for trials from Cerenis Therapeutics, The Medicines Company, and Esperion; advisory board fees from Akcea Therapeutics, Novartis, Silence Therapeutics, Bayer, and Daiichi Sankyo; lecture fees and advisory board fees from Takeda, Boehringer Ingelheim, and Dr Reddy’s Laboratories; grant support and advisory board fees from Merck Sharp & Dohme; fees for serving on a clinical events adjudication committee from AbbVie; and fees for serving as principal investigator for a trial from Resverlogix; Z.T., S.V., and X.Z. are all employees of Novartis Pharmaceuticals Corp, East Hanover, New Jersey, USA and hold shares in Novartis; A.L. is employee of Novartis Pharma AG, Basel, Switzerland; P.M. was an employee of Novartis Pharma AG, Basel, Switzerland, at the time of conducting this study.
Figures






Comment in
-
ORION-8: one step closer to understanding the safety and efficacy of inclisiran.Cardiovasc Res. 2024 Oct 14;120(12):1365-1366. doi: 10.1093/cvr/cvae166. Cardiovasc Res. 2024. PMID: 39082271 No abstract available.
References
-
- Khvorova A. Oligonucleotide therapeutics -a new class of cholesterol-lowering drugs. N Engl J Med 2017;376:4–7. - PubMed
-
- Wright RS, Ray KK, Raal FJ, Kallend DG, Jaros M, Koenig W, Leiter LA, Landmesser U, Schwartz GG, Friedman A, Wijngaard PLJ, Garcia Conde L, Kastelein JJP, Investigators OPI. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol 2021;77:1182–1193. - PubMed
-
- Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, Kastelein JJP. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med 2020;382:1520–1530. - PubMed
-
- Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–1519. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

