Ultrapotent influenza hemagglutinin fusion inhibitors developed through SuFEx-enabled high-throughput medicinal chemistry
- PMID: 38753503
- PMCID: PMC11145270
- DOI: 10.1073/pnas.2310677121
Ultrapotent influenza hemagglutinin fusion inhibitors developed through SuFEx-enabled high-throughput medicinal chemistry
Abstract
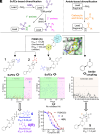
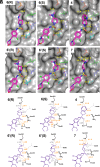
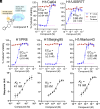
Seasonal and pandemic-associated influenza strains cause highly contagious viral respiratory infections that can lead to severe illness and excess mortality. Here, we report on the optimization of our small-molecule inhibitor F0045(S) targeting the influenza hemagglutinin (HA) stem with our Sulfur-Fluoride Exchange (SuFEx) click chemistry-based high-throughput medicinal chemistry (HTMC) strategy. A combination of SuFEx- and amide-based lead molecule diversification and structure-guided design led to identification and validation of ultrapotent influenza fusion inhibitors with subnanomolar EC50 cellular antiviral activity against several influenza A group 1 strains. X-ray structures of six of these compounds with HA indicate that the appended moieties occupy additional pockets on the HA surface and increase the binding interaction, where the accumulation of several polar interactions also contributes to the improved affinity. The compounds here represent the most potent HA small-molecule inhibitors to date. Our divergent HTMC platform is therefore a powerful, rapid, and cost-effective approach to develop bioactive chemical probes and drug-like candidates against viral targets.
Keywords: Sulfur-Fluoride Exchange; click chemistry; high-throughput screening; influenza hemagglutinin inhibitor; x-ray crystallography.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
SuFEx-enabled high-throughput medicinal chemistry for developing potent tamoxifen analogs as Ebola virus entry inhibitors.Front Immunol. 2025 Apr 28;16:1533037. doi: 10.3389/fimmu.2025.1533037. eCollection 2025. Front Immunol. 2025. PMID: 40356906 Free PMC article.
-
An influenza A hemagglutinin small-molecule fusion inhibitor identified by a new high-throughput fluorescence polarization screen.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18431-18438. doi: 10.1073/pnas.2006893117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690700 Free PMC article.
-
Design of inhibitors of influenza virus membrane fusion: synthesis, structure-activity relationship and in vitro antiviral activity of a novel indole series.Antiviral Res. 2013 Aug;99(2):125-35. doi: 10.1016/j.antiviral.2013.05.005. Epub 2013 May 22. Antiviral Res. 2013. PMID: 23707194
-
Membrane Fusion and Infection of the Influenza Hemagglutinin.Adv Exp Med Biol. 2017;966:37-54. doi: 10.1007/5584_2016_174. Adv Exp Med Biol. 2017. PMID: 27966108 Review.
-
Influenza A virus entry inhibitors targeting the hemagglutinin.Viruses. 2013 Jan 22;5(1):352-73. doi: 10.3390/v5010352. Viruses. 2013. PMID: 23340380 Free PMC article. Review.
Cited by
-
SuFEx-enabled high-throughput medicinal chemistry for developing potent tamoxifen analogs as Ebola virus entry inhibitors.Front Immunol. 2025 Apr 28;16:1533037. doi: 10.3389/fimmu.2025.1533037. eCollection 2025. Front Immunol. 2025. PMID: 40356906 Free PMC article.
References
-
- Molinari N. A., et al. , The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 25, 5086–5096 (2007). - PubMed
-
- Belongia E. A., et al. , Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 16, 942–951 (2016). - PubMed
MeSH terms
Grants and funding
- K99 GM138758/GM/NIGMS NIH HHS/United States
- K99GM138758/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 AI150885/AI/NIAID NIH HHS/United States
- R01AI150885/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R35GM136286/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources

