Essential role of N-terminal SAM regions in STIM1 multimerization and function
- PMID: 38753510
- PMCID: PMC11127010
- DOI: 10.1073/pnas.2318874121
Essential role of N-terminal SAM regions in STIM1 multimerization and function
Abstract
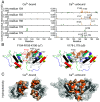
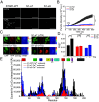
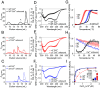
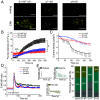
The single-pass transmembrane protein Stromal Interaction Molecule 1 (STIM1), located in the endoplasmic reticulum (ER) membrane, possesses two main functions: It senses the ER-Ca2+ concentration and directly binds to the store-operated Ca2+ channel Orai1 for its activation when Ca2+ recedes. At high resting ER-Ca2+ concentration, the ER-luminal STIM1 domain is kept monomeric but undergoes di/multimerization once stores are depleted. Luminal STIM1 multimerization is essential to unleash the STIM C-terminal binding site for Orai1 channels. However, structural basis of the luminal association sites has so far been elusive. Here, we employed molecular dynamics (MD) simulations and identified two essential di/multimerization segments, the α7 and the adjacent region near the α9-helix in the sterile alpha motif (SAM) domain. Based on MD results, we targeted the two STIM1 SAM domains by engineering point mutations. These mutations interfered with higher-order multimerization of ER-luminal fragments in biochemical assays and puncta formation in live-cell experiments upon Ca2+ store depletion. The STIM1 multimerization impeded mutants significantly reduced Ca2+ entry via Orai1, decreasing the Ca2+ oscillation frequency as well as store-operated Ca2+ entry. Combination of the ER-luminal STIM1 multimerization mutations with gain of function mutations and coexpression of Orai1 partially ameliorated functional defects. Our data point to a hydrophobicity-driven binding within the ER-luminal STIM1 multimer that needs to switch between resting monomeric and activated multimeric state. Altogether, these data reveal that interactions between SAM domains of STIM1 monomers are critical for multimerization and activation of the protein.
Keywords: EF-SAM; Orai; SOCE; STIM.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Brini M., Ottolini D., Cali T., Carafoli E., Calcium in health and disease. Met Ions Life Sci. 13, 81–137 (2013). - PubMed
-
- Berridge M. J., Bootman M. D., Roderick H. L., Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 (2003). - PubMed
-
- Putney J. W. Jr., A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12 (1986). - PubMed
-
- Emrich S. M., Yoast R. E., Trebak M., Physiological functions of CRAC channels. Annu. Rev. Physiol. 84, 355–379 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

