Exploiting bacterial effector proteins to uncover evolutionarily conserved antiviral host machinery
- PMID: 38753575
- PMCID: PMC11098378
- DOI: 10.1371/journal.ppat.1012010
Exploiting bacterial effector proteins to uncover evolutionarily conserved antiviral host machinery
Abstract
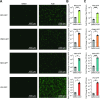
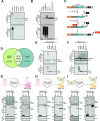
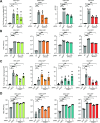
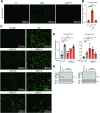
Arboviruses are a diverse group of insect-transmitted pathogens that pose global public health challenges. Identifying evolutionarily conserved host factors that combat arbovirus replication in disparate eukaryotic hosts is important as they may tip the balance between productive and abortive viral replication, and thus determine virus host range. Here, we exploit naturally abortive arbovirus infections that we identified in lepidopteran cells and use bacterial effector proteins to uncover host factors restricting arbovirus replication. Bacterial effectors are proteins secreted by pathogenic bacteria into eukaryotic hosts cells that can inhibit antimicrobial defenses. Since bacteria and viruses can encounter common host defenses, we hypothesized that some bacterial effectors may inhibit host factors that restrict arbovirus replication in lepidopteran cells. Thus, we used bacterial effectors as molecular tools to identify host factors that restrict four distinct arboviruses in lepidopteran cells. By screening 210 effectors encoded by seven different bacterial pathogens, we identify several effectors that individually rescue the replication of all four arboviruses. We show that these effectors encode diverse enzymatic activities that are required to break arbovirus restriction. We further characterize Shigella flexneri-encoded IpaH4 as an E3 ubiquitin ligase that directly ubiquitinates two evolutionarily conserved proteins, SHOC2 and PSMC1, promoting their degradation in insect and human cells. We show that depletion of either SHOC2 or PSMC1 in insect or human cells promotes arbovirus replication, indicating that these are ancient virus restriction factors conserved across invertebrate and vertebrate hosts. Collectively, our study reveals a novel pathogen-guided approach to identify conserved antimicrobial machinery, new effector functions, and conserved roles for SHOC2 and PSMC1 in virus restriction.
Copyright: © 2024 Embry et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures



Update of
-
Exploiting Bacterial Effector Proteins to Uncover Evolutionarily Conserved Antiviral Host Machinery.bioRxiv [Preprint]. 2024 Jan 30:2024.01.29.577891. doi: 10.1101/2024.01.29.577891. bioRxiv. 2024. Update in: PLoS Pathog. 2024 May 16;20(5):e1012010. doi: 10.1371/journal.ppat.1012010. PMID: 38352400 Free PMC article. Updated. Preprint.
Similar articles
-
Bacterial effector screening reveals RNF214 as a virus restriction factor in mammals.PLoS Pathog. 2025 Apr 22;21(4):e1013035. doi: 10.1371/journal.ppat.1013035. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40261845 Free PMC article.
-
Exploiting Bacterial Effector Proteins to Uncover Evolutionarily Conserved Antiviral Host Machinery.bioRxiv [Preprint]. 2024 Jan 30:2024.01.29.577891. doi: 10.1101/2024.01.29.577891. bioRxiv. 2024. Update in: PLoS Pathog. 2024 May 16;20(5):e1012010. doi: 10.1371/journal.ppat.1012010. PMID: 38352400 Free PMC article. Updated. Preprint.
-
Bacterial Effector Screening Reveals RNF214 as a Virus Restriction Factor in Mammals.bioRxiv [Preprint]. 2024 Nov 4:2024.11.04.621956. doi: 10.1101/2024.11.04.621956. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Apr 22;21(4):e1013035. doi: 10.1371/journal.ppat.1013035. PMID: 39574573 Free PMC article. Updated. Preprint.
-
The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses.Philos Trans R Soc Lond B Biol Sci. 2015 Aug 19;370(1675):20140299. doi: 10.1098/rstb.2014.0299. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26150665 Free PMC article. Review.
-
Arbovirus-mosquito interactions: RNAi pathway.Curr Opin Virol. 2015 Dec;15:119-26. doi: 10.1016/j.coviro.2015.10.001. Epub 2015 Dec 6. Curr Opin Virol. 2015. PMID: 26629932 Free PMC article. Review.
Cited by
-
Bacterial effector screening reveals RNF214 as a virus restriction factor in mammals.PLoS Pathog. 2025 Apr 22;21(4):e1013035. doi: 10.1371/journal.ppat.1013035. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40261845 Free PMC article.
-
Nucleolar beacon for monitoring nucleolar morphology and proteomics in living cells.Sci Adv. 2025 Jun 13;11(24):eadv7795. doi: 10.1126/sciadv.adv7795. Epub 2025 Jun 11. Sci Adv. 2025. PMID: 40498842 Free PMC article.
-
Activation and Evasion of the FEAR Pathway by RNA Viruses.bioRxiv [Preprint]. 2025 Feb 25:2024.08.22.609092. doi: 10.1101/2024.08.22.609092. bioRxiv. 2025. PMID: 40060670 Free PMC article. Preprint.
References
-
- Velazquez-Salinas L, Pauszek SJ, Stenfeldt C, O’Hearn ES, Pacheco JM, Borca MV, et al.. Increased Virulence of an Epidemic Strain of Vesicular Stomatitis Virus Is Associated With Interference of the Innate Response in Pigs. Front Microbiol. 2018;9:1891. Epub 2018/08/31. doi: 10.3389/fmicb.2018.01891 ; PubMed Central PMCID: PMC6104175. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

