Muscle Psn gene combined with exercise contribute to healthy aging of skeletal muscle and lifespan by adaptively regulating Sirt1/PGC-1α and arm pathway
- PMID: 38753634
- PMCID: PMC11098322
- DOI: 10.1371/journal.pone.0300787
Muscle Psn gene combined with exercise contribute to healthy aging of skeletal muscle and lifespan by adaptively regulating Sirt1/PGC-1α and arm pathway
Abstract
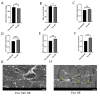
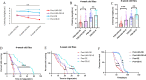
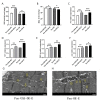
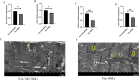
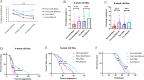
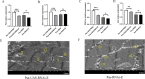
The Presenilin (Psn) gene is closely related to aging, but it is still unclear the role of Psn genes in skeletal muscle. Here, the Psn-UAS/Mhc-GAL4 system in Drosophila was used to regulate muscle Psn overexpression(MPO) and muscle Psn knockdown(MPK). Drosophila were subjected to endurance exercise from 4 weeks to 5 weeks old. The results showed that MPO and exercise significantly increased climbing speed, climbing endurance, lifespan, muscle SOD activity, Psn expression, Sirt1 expression, PGC-1α expression, and armadillo (arm) expression in aged Drosophila, and they significantly decreased muscle malondialdehyde levels. Interestingly, when the Psn gene is knockdown by 0.78 times, the PGC-1α expression and arm expression were also down-regulated, but the exercise capacity and lifespan were increased. Furthermore, exercise combined with MPO further improved the exercise capacity and lifespan. MPK combined with exercise further improves the exercise capacity and lifespan. Thus, current results confirmed that the muscle Psn gene was a vital gene that contributed to the healthy aging of skeletal muscle since whether it was overexpressed or knocked down, the aging progress of skeletal muscle structure and function was slowed down by regulating the activity homeostasis of Sirt1/PGC-1α pathway and Psn/arm pathway. Exercise enhanced the function of the Psn gene to delay skeletal muscle aging by up regulating the activity of the Sirt1/PGC-1α pathway and Psn/arm pathway.
Copyright: © 2024 Gao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Muscle-specific overexpression of Atg2 gene and endurance exercise delay age-related deteriorations of skeletal muscle and heart function via activating the AMPK/Sirt1/PGC-1α pathway in male Drosophila.FASEB J. 2023 Nov;37(11):e23214. doi: 10.1096/fj.202301312R. FASEB J. 2023. PMID: 37773768
-
Physical exercise ameliorates age-related deterioration of skeletal muscle and mortality by activating Pten-related pathways in Drosophila on a high-salt diet.FASEB J. 2023 Dec;37(12):e23304. doi: 10.1096/fj.202301099R. FASEB J. 2023. PMID: 37971426
-
Effect of Exercise Training on Skeletal Muscle SIRT1 and PGC-1α Expression Levels in Rats of Different Age.Int J Med Sci. 2016 Mar 16;13(4):260-70. doi: 10.7150/ijms.14586. eCollection 2016. Int J Med Sci. 2016. PMID: 27076782 Free PMC article.
-
Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis.Appl Physiol Nutr Metab. 2011 Oct;36(5):589-97. doi: 10.1139/h11-070. Epub 2011 Sep 2. Appl Physiol Nutr Metab. 2011. PMID: 21888529 Review.
-
PGC-1alpha-mediated adaptations in skeletal muscle.Pflugers Arch. 2010 Jun;460(1):153-62. doi: 10.1007/s00424-010-0834-0. Epub 2010 Apr 19. Pflugers Arch. 2010. PMID: 20401754 Review.
Cited by
-
Endurance exercise attenuates Gαq-RNAi induced hereditary obesity and skeletal muscle dysfunction via improving skeletal muscle Srl/MRCC-I pathway in Drosophila.Sci Rep. 2024 Nov 15;14(1):28207. doi: 10.1038/s41598-024-79415-x. Sci Rep. 2024. PMID: 39548180 Free PMC article.
-
Structural insights into a highly flexible zinc finger module unravel INSM1 function in transcription regulation.Nat Commun. 2025 Mar 4;16(1):2162. doi: 10.1038/s41467-025-57478-2. Nat Commun. 2025. PMID: 40038295 Free PMC article.
-
Elucidation of anti-human melanoma and anti-aging mechanisms of compounds from green seaweed Caulerpa racemosa.Sci Rep. 2024 Nov 11;14(1):27534. doi: 10.1038/s41598-024-78464-6. Sci Rep. 2024. PMID: 39528552 Free PMC article.
References
-
- Gao Y.H., Wen D.T., Wang S.J., Wang J.F., Presenilin and Alzheimer’s disease interactions with aging, exercise and high-fat diet: A systematic review, Biocell 47(1) (2023) 41–49.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

