Retinoic acid alleviates the reduction of Akt and Bad phosphorylation and regulates Bcl-2 family protein interactions in animal models of ischemic stroke
- PMID: 38753710
- PMCID: PMC11098415
- DOI: 10.1371/journal.pone.0303213
Retinoic acid alleviates the reduction of Akt and Bad phosphorylation and regulates Bcl-2 family protein interactions in animal models of ischemic stroke
Abstract
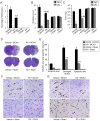
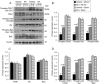
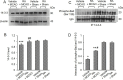
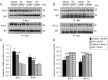
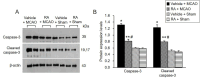
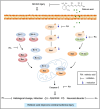
Ischemic stroke causes a lack of oxygen and glucose supply to brain, eventually leads to severe neurological disorders. Retinoic acid is a major metabolic product of vitamin A and has various biological effects. The PI3K-Akt signaling pathway is an important survival pathway in brain. Phosphorylated Akt is important in regulating survival and apoptosis. We examined whether retinoic acid has neuroprotective effects in stroke model by regulating Akt and its downstream protein, Bad. Moreover, we investigated the relationship between retinoic acid and Bcl-2 family protein interactions. Animals were intraperitoneally administered vehicle or retinoic acid (5 mg/kg) for four days before surgery and ischemic stroke was induced by middle cerebral artery occlusion (MCAO) surgery. Neurobehavioral tests were performed 24 h after MCAO and cerebral cortical tissues were collected. Cresyl violet staining and TUNEL histochemistry were performed, Western blot and immunoprecipitation analysis were performed to elucidate the expression of various proteins. Retinoic acid reduced neurological deficits and histopathological changes, decreased the number of TUNEL-positive cells, and alleviated reduction of phospho-PDK1, phospho-Akt, and phospho-Bad expression caused by MCAO damage. Immunoprecipitation analysis showed that MCAO damage reduced the interaction between phospho-Bad and 14-3-3, which was attenuated by retinoic acid. Furthermore, retinoic acid mitigated the increase in Bcl-2/Bad and Bcl-xL/Bad binding levels and the reduction in Bcl-2/Bax and Bcl-xL/Bax binding levels caused by MCAO damage. Retinoic acid alleviated MCAO-induced increase of caspase-3 and cleaved caspase-3 expression. We demonstrate that retinoic acid prevented apoptosis against cerebral ischemia through phosphorylation of Akt and Bad, maintenance of phospho-Bad and 14-3-3 binding, and regulation of Bcl-2 family protein interactions. .
Copyright: © 2024 Kang, Koh. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Chlorogenic acid alleviates the reduction of Akt and Bad phosphorylation and of phospho-Bad and 14-3-3 binding in an animal model of stroke.J Vet Sci. 2022 Nov;23(6):e84. doi: 10.4142/jvs.22200. Epub 2022 Sep 30. J Vet Sci. 2022. PMID: 36259103 Free PMC article.
-
Retinoic acid exerts neuroprotective effects against focal cerebral ischemia by preventing apoptotic cell death.Neurosci Lett. 2021 Jul 13;757:135979. doi: 10.1016/j.neulet.2021.135979. Epub 2021 May 20. Neurosci Lett. 2021. PMID: 34023410
-
Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation.Neurosci Lett. 2012 Jan 24;507(2):156-60. doi: 10.1016/j.neulet.2011.12.012. Epub 2011 Dec 17. Neurosci Lett. 2012. PMID: 22200499
-
Retinoic Acid Has Neuroprotective effects by Modulating Thioredoxin in Ischemic Brain Damage and Glutamate-exposed Neurons.Neuroscience. 2023 Jun 15;521:166-181. doi: 10.1016/j.neuroscience.2023.04.028. Epub 2023 May 4. Neuroscience. 2023. PMID: 37149281
-
Retinoic Acid Prevents the Neuronal Damage Through the Regulation of Parvalbumin in an Ischemic Stroke Model.Neurochem Res. 2023 Feb;48(2):487-501. doi: 10.1007/s11064-022-03769-9. Epub 2022 Oct 16. Neurochem Res. 2023. PMID: 36245066
Cited by
-
Curcumin ameliorates heatstroke-induced lung injury by activating the PI3K/AKT pathway.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):4617-4632. doi: 10.1007/s00210-024-03572-z. Epub 2024 Nov 9. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39521756
-
Neuroinflammation and energy metabolism: a dual perspective on ischemic stroke.J Transl Med. 2025 Apr 10;23(1):413. doi: 10.1186/s12967-025-06440-3. J Transl Med. 2025. PMID: 40211331 Free PMC article. Review.
-
Amyotrophic Lateral Sclerosis and Parkinson's Disease: Brain Tissue Transcriptome Analysis Reveals Interactions.Mol Neurobiol. 2025 May;62(5):6383-6396. doi: 10.1007/s12035-024-04681-9. Epub 2025 Jan 10. Mol Neurobiol. 2025. PMID: 39792201 Review.
-
Role of miRNAs in Apoptosis Pathways of Immune Cells in Systemic Lupus Erythematosus.Immun Inflamm Dis. 2025 Feb;13(2):e70124. doi: 10.1002/iid3.70124. Immun Inflamm Dis. 2025. PMID: 39912562 Free PMC article. Review.
-
Electroacupuncture alleviated post-stroke cognitive impairment via the mTOR/NLRP3-mediated autophagy-inflammatory pathway.Eur J Med Res. 2024 Nov 5;29(1):532. doi: 10.1186/s40001-024-02131-9. Eur J Med Res. 2024. PMID: 39497200 Free PMC article.
References
-
- García-Regalado A, Vargas M, García-Carrancá A, Aréchaga-Ocampo E, González-De la Rosa CH. Activation of Akt pathway by transcription-independent mechanisms of retinoic acid promotes survival and invasion in lung cancer cells. Mol Cancer. 2013;12: 44. doi: 10.1186/1476-4598-12-44 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

