Navigating regulatory landscape: A qualitative exploration of medical devices and in vitro diagnostic medical devices oversight in Zimbabwe through key stakeholder perspectives
- PMID: 38753856
- PMCID: PMC11098438
- DOI: 10.1371/journal.pone.0287415
Navigating regulatory landscape: A qualitative exploration of medical devices and in vitro diagnostic medical devices oversight in Zimbabwe through key stakeholder perspectives
Abstract
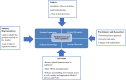
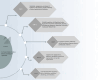
Medical devices and In Vitro Diagnostics (IVDs) are vital for public health and accessible healthcare. Still, there is an imbalance in high-quality products in Low and Middle-Income Countries (LMICs). Zimbabwe's regulatory framework for medical devices and IVDs is unclear, leading to ineffective compliance and surveillance. As a result, there are knowledge gaps regarding pre-market and post-market regulatory elements to ensure the safety, quality and performance of medical devices and IVDs used in Zimbabwe. Our study aimed to explore the current status of medical devices and IVD regulations in Zimbabwe. Semi-structured interviews were conducted with 12 regulators from the Medicines Control Authority of Zimbabwe (MCAZ) National Microbiology Reference Laboratory (NMRL), Medical Laboratory and Clinical Scientists Council (MLCScCZ) to understand the current status of medical devices and IVD regulations in Zimbabwe. Three participants completed a questionnaire to understand the regulatory landscape in Zimbabwe. Three key informant interviews were conducted with three regulators from the South African Health Products Regulatory Authority (SAHPRA), Tanzanian Medicines and Medical Devices Authority (TMDA), and World Health Organization Regulatory Systems Strengthening (WHO RSS) to learn best practices to create a roadmap for Zimbabwe. We analyzed qualitative data using a thematic analysis. The findings reveal significant deficiencies and gaps in the legal framework for regulating medical devices and IVDs, highlighting the need for a legal framework and the absence of more comprehensive regulations. Regulatory entities face capacity limitations, especially in regulating medical devices and IVDs. Conformity assessment processes, medical devices, IVD classification criteria, and post-market surveillance also represent challenges, highlighting the need for a well-defined framework and regulatory procedures. The Zimbabwean regulatory system pathway is reactive, prompting several regulatory initiatives to address needs. Despite facing challenges, there is recognition of the importance of collaboration among regulatory authorities, emphasizing a shared commitment to improving and strengthening medical devices and IVD regulations for improved patient safety. By advocating for a proactive, comprehensive, and legally sound approach, indicating the potential for collaboration and synergy, this study provides a foundation for well-informed policy recommendations to guide enhancements and build a framework for a resilient, efficient, and transparent regulatory environment in the Zimbabwe and African regions as a whole.
Copyright: © 2024 Chiku et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Assessing operational readiness: Regulatory landscape and compliance in zimbabwe for medical devices and in vitro diagnostic medical devices.PLoS One. 2024 May 16;19(5):e0287495. doi: 10.1371/journal.pone.0287495. eCollection 2024. PLoS One. 2024. PMID: 38753597 Free PMC article.
-
Regulation of HIV self-testing in Malawi, Zambia and Zimbabwe: a qualitative study with key stakeholders.J Int AIDS Soc. 2019 Mar;22 Suppl 1(Suppl Suppl 1):e25229. doi: 10.1002/jia2.25229. J Int AIDS Soc. 2019. PMID: 30907513 Free PMC article.
-
Regulation of medical diagnostics and medical devices in the East African community partner states.BMC Health Serv Res. 2014 Oct 31;14:524. doi: 10.1186/s12913-014-0524-2. BMC Health Serv Res. 2014. PMID: 25366990 Free PMC article.
-
[Diagnostic kits in parasitology: which controls?].Parassitologia. 2004 Jun;46(1-2):145-9. Parassitologia. 2004. PMID: 15305705 Review. Italian.
-
Access and reimbursement pathways for digital health solutions and in vitro diagnostic devices: Current scenario and challenges.Front Med Technol. 2023 Feb 20;5:1101476. doi: 10.3389/fmedt.2023.1101476. eCollection 2023. Front Med Technol. 2023. PMID: 36891483 Free PMC article. Review.
References
-
- European Commission. MDR—Regulation (EU) 2017/746 on in-vitro diagnostic medical devices. Off J Eur Union; [Internet]. 2017;60(April 2014):1–175. Available from: 1. European Commission. MDR—Regulation (EU) 2017/746 on in-vitro diagnostic medical devices. Off J Eur Union [Internet]. 2017;60(April 2014):1–175. Available from: https://www.emergogroup.com/sites/default/files/europe-medical-devices-r...
-
- (MDCG) MDCG. Joint implementation and preparedness plan for Regulation (EU) 2017 / 746 on in vitro diagnostic medical devices (IVDR). 2022;(April 2020):18–22. Available from: https://health.ec.europa.eu/latest-updates/updated-joint-implementation-...
-
- REGULATORY AFFAIRS PROFESSIONALS SOCIETY. Fundamentals_of_Medical_Device_Regulations__Fifth_.pdf. Fifth. Hall GN, editor. Rockville: RAPS BOOKS; 2022. 748 p.
-
- GHTF. Global Harmonization Task Force Study Group 1: Definition of the Terms ‘Medical Device’ and ‘In Vitro Diagnostic (IVD) Medical Device.’ Force, Study Gr 1 Glob Harmon Task [Internet]. 2012;(Ivd):6. Available from: http://www.imdrf.org/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n071-20... definition ?Medical Device? 2012%22
-
- GHTF. Essential principles of safety and performance of medical devies. 2012;1–24. Available from: http://www.imdrf.org/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n68-201...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

