Comparative effectiveness of denosumab vs alendronate among postmenopausal women with osteoporosis
- PMID: 38753892
- PMCID: PMC11301726
- DOI: 10.1093/jbmr/zjae079
Comparative effectiveness of denosumab vs alendronate among postmenopausal women with osteoporosis
Abstract
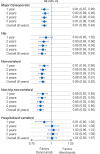
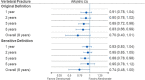
Although clinical trials have shown that denosumab significantly increases bone mineral density at key skeletal sites more than oral bisphosphonates, evidence is lacking from head-to-head randomized trials evaluating fracture outcomes. This retrospective cohort study uses administrative claims data from Medicare fee-for service beneficiaries to evaluate the comparative effectiveness of denosumab vs alendronate in reducing fracture risk among women with PMO in the US. Women with PMO ≥ 66 yr of age with no prior history of osteoporosis treatment, who initiated denosumab (n = 89 115) or alendronate (n = 389 536) from 2012 to 2018, were followed from treatment initiation until the first of a specific fracture outcome, treatment discontinuation or switch, end of study (December 31, 2019), or other censoring criteria. A doubly robust inverse-probability of treatment and censoring weighted function was used to estimate the risk ratio associated with the use of denosumab compared with alendronate for hip, nonvertebral (NV; includes hip, humerus, pelvis, radius/ulna, other femur), non-hip nonvertebral (NHNV), hospitalized vertebral (HV), and major osteoporotic (MOP; consisting of NV and HV) fractures. Overall, denosumab reduced the risk of MOP by 39%, hip by 36%, NV by 43%, NHNV by 50%, and HV fractures by 30% compared with alendronate. Denosumab reduced the risk of MOP fractures by 9% at year 1, 12% at year 2, 18% at year 3, and 31% at year 5. An increase in the magnitude of fracture risk reduction with increasing duration of exposure was also observed for other NV fracture outcomes. In this cohort of almost half-a-million treatment-naive women with PMO, we observed clinically significant reductions in the risk of MOP, hip, NV, NHNV, and HV fractures for patients on denosumab compared with alendronate. Patients who remained on denosumab for longer periods of time experienced greater reductions in fracture risk.
Keywords: antiresorptives; fracture prevention; general population studies; osteoporosis; statistical methods.
Plain language summary
Osteoporosis-related fractures can have a significant impact on the health and quality of life of women with postmenopausal osteoporosis, as well as pose a significant burden to society. Although clinical trials have shown that denosumab is more effective at increasing bone mineral density compared with alendronate, there is a lack of evidence evaluating the fracture risk between these 2 commonly used osteoporosis therapies. In this study using Medicare claims data for almost 500 000 women with postmenopausal osteoporosis with no prior history of osteoporosis medication use, we compared the risk of fracture—an important outcome to patients and health care providers—between denosumab and alendronate. Advanced analytic methods were implemented to ensure the study results were valid and were not unduly influenced by biases common in observational studies. We observed clinically meaningful reductions (from 30% up to 50%) in the risk of hip, nonvertebral, non-hip nonvertebral, hospitalized vertebral, and major osteoporotic fractures for patients treated with denosumab compared with alendronate. Patients who remained on denosumab for longer periods of time experienced greater reductions in fracture risk than those who remained on alendronate.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
J.R.C. has received consulting fees and research grants from Amgen.
T.A. has received research grants from Amgen.
Y.L. has received research grants from Amgen.
T.-C.L is an employee and owns equity in Amgen.
L.S. is an employee and owns equity in Amgen.
V.C.B. is an employee and owns equity in Amgen.
R.K.S. is a former employee and owns equity in Amgen.
M.McD. is an employee and owns equity in Amgen.
B.D.B. is an employee and owns equity in Amgen.
M.K. is an employee and owns equity in Amgen.
Figures


References
-
- Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1-2):136. 10.1007/s11657-013-0136-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

