TEMPORAL CHANGES IN INNATE AND ADAPTIVE IMMUNITY DURING SEPSIS AS DETERMINED BY ELISPOT
- PMID: 38754032
- PMCID: PMC11348958
- DOI: 10.1097/SHK.0000000000002377
TEMPORAL CHANGES IN INNATE AND ADAPTIVE IMMUNITY DURING SEPSIS AS DETERMINED BY ELISPOT
Abstract
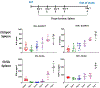
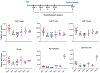
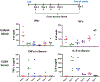
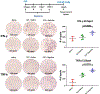
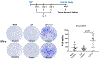
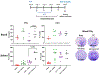
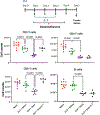
Background: The inability to evaluate host immunity in a rapid quantitative manner in patients with sepsis has severely hampered development of novel immune therapies. The enzyme-linked immunospot (ELISpot) assay is a functional bioassay that measures the number of cytokine-secreting cells and the relative amount of cytokine produced at the single-cell level. A key advantage of ELISpot is its excellent dynamic range enabling a more precise quantifiable assessment of host immunity. Herein, we tested the hypothesis that the ELISpot assay can detect dynamic changes in both innate and adaptive immunity as they often occur during sepsis. We also tested whether ELISpot could detect the effect of immune drug therapies to modulate innate and adaptive immunity. Methods: Mice were made septic using sublethal cecal ligation and puncture. Blood and spleens were harvested serially, and ex vivo interferon γ and TNF-α production were compared by ELISpot and enzyme-linked immunosorbent assay. The capability of ELISpot to detect changes in innate and adaptive immunity due to in vivo immune therapy with dexamethasone, IL-7, and arginine was also evaluated. Results: ELISpot confirmed a decreased innate and adaptive immunity responsiveness during sepsis progression. More importantly, ELISpot was also able to detect changes in adaptive and innate immunity in response to immune-modulatory reagents, for example, dexamethasone, arginine, and IL-7, in a readily quantifiable manner, as predicted by the reagents known mechanisms of action. ELISpot and enzyme-linked immunosorbent assay results tended to parallel one another although some differences were noted. Conclusion: ELISpot offers a unique capability to assess the functional status of both adaptive and innate immunity over time. The results presented herein demonstrate that ELISpot can also be used to detect and follow the in vivo effects of drugs to ameliorate sepsis-induced immune dysfunction. This capability would be a major advance in guiding new immune therapies in sepsis.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Shock Society.
Figures

Update of
-
Temporal Changes in Innate and Adaptive Immunity During Sepsis as Determined by ELISpot.bioRxiv [Preprint]. 2023 Dec 14:2023.12.14.571668. doi: 10.1101/2023.12.14.571668. bioRxiv. 2023. Update in: Shock. 2024 Aug 1;62(2):255-264. doi: 10.1097/SHK.0000000000002377. PMID: 38168302 Free PMC article. Updated. Preprint.
Similar articles
-
Determining potential immunomodulatory drug efficacy in sepsis using ELISpot.Sci Rep. 2025 Apr 18;15(1):13464. doi: 10.1038/s41598-025-92016-6. Sci Rep. 2025. PMID: 40251188 Free PMC article.
-
Temporal Changes in Innate and Adaptive Immunity During Sepsis as Determined by ELISpot.bioRxiv [Preprint]. 2023 Dec 14:2023.12.14.571668. doi: 10.1101/2023.12.14.571668. bioRxiv. 2023. Update in: Shock. 2024 Aug 1;62(2):255-264. doi: 10.1097/SHK.0000000000002377. PMID: 38168302 Free PMC article. Updated. Preprint.
-
Development and optimization of a diluted whole blood ELISpot assay to test immune function.J Immunol Methods. 2024 Oct;533:113743. doi: 10.1016/j.jim.2024.113743. Epub 2024 Aug 13. J Immunol Methods. 2024. PMID: 39147231
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Determining potential immunomodulatory drug efficacy in sepsis using ELISpot.Sci Rep. 2025 Apr 18;15(1):13464. doi: 10.1038/s41598-025-92016-6. Sci Rep. 2025. PMID: 40251188 Free PMC article.
-
New Criteria for Pediatric Sepsis: A Phoenix Rising.J Pediatr Pharmacol Ther. 2024 Dec;29(6):676-678. doi: 10.5863/1551-6776-29.6.676. Epub 2024 Dec 9. J Pediatr Pharmacol Ther. 2024. PMID: 39659861 Free PMC article. No abstract available.
References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. Aug 29 2013;369(9):840–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

