Mutational profile in previously treated patients with chronic lymphocytic leukemia progression on acalabrutinib or ibrutinib
- PMID: 38754046
- PMCID: PMC11406168
- DOI: 10.1182/blood.2023023659
Mutational profile in previously treated patients with chronic lymphocytic leukemia progression on acalabrutinib or ibrutinib
Abstract
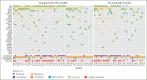
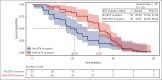
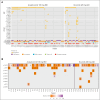
Chronic lymphocytic leukemia (CLL) progression during Bruton tyrosine kinase (BTK) inhibitor treatment is typically characterized by emergent B-cell receptor pathway mutations. Using peripheral blood samples from patients with relapsed/refractory CLL in ELEVATE-RR (NCT02477696; median 2 prior therapies), we report clonal evolution data for patients progressing on acalabrutinib or ibrutinib (median follow-up, 41 months). Paired (baseline and progression) samples were available for 47 (excluding 1 Richter) acalabrutinib-treated and 30 (excluding 6 Richter) ibrutinib-treated patients. At progression, emergent BTK mutations were observed in 31 acalabrutinib-treated (66%) and 11 ibrutinib-treated patients (37%; median variant allele fraction [VAF], 16.1% vs 15.6%, respectively). BTK C481S mutations were most common in both groups; T474I (n = 9; 8 co-occurring with C481) and the novel E41V mutation within the pleckstrin homology domain of BTK (n = 1) occurred with acalabrutinib, whereas neither mutation occurred with ibrutinib. L528W and A428D comutations presented in 1 ibrutinib-treated patient. Preexisting TP53 mutations were present in 25 acalabrutinib-treated (53.2%) and 16 ibrutinib-treated patients (53.3%) at screening. Emergent TP53 mutations occurred with acalabrutinib and ibrutinib (13% vs 7%; median VAF, 6.0% vs 37.3%, respectively). Six acalabrutinib-treated patients and 1 ibrutinib-treated patient had emergent TP53/BTK comutations. Emergent PLCG2 mutations occurred in 3 acalabrutinib-treated (6%) and 6 ibrutinib-treated patients (20%). One acalabrutinib-treated patient and 4 ibrutinib-treated patients had emergent BTK/PLCG2 comutations. Although common BTK C481 mutations were observed with both treatments, patterns of mutation and comutation frequency, mutation VAF, and uncommon BTK variants varied with acalabrutinib (T474I and E41V) and ibrutinib (L528W and A428D) in this patient population. The trial was registered at www.clinicaltrials.gov as #NCT02477696.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.A.W. reports research funding from AbbVie, Janssen, Karyopharm Therapeutics, Loxo/Lilly, Pharmacyclics, and Schrodinger; holds a consultant or advisory role for AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Merck, Loxo/Lilly, Newave, and Pharmacyclics. D.J. reports research funding from AbbVie, Acerta/AstraZeneca, Pharmacyclics, Novartis, and MingSight; and support from The Ohio State University for high sensitivity Bruton tyrosine kinase mutation profiling. W.J. reports research funding from AbbVie, AstraZeneca, BeiGene, Janssen, Lilly, Roche, and Takeda; and consultant or advisory role fees from AbbVie, AstraZeneca, BeiGene, Lilly, Roche, and Takeda. T.R. reports research funding, consultant or advisory role fees, and honoraria from AstraZeneca, BeiGene, and Janssen. Á.I. reports research funding from Takeda and Seattle Genetics; and honoraria from Janssen, Celgene, Novartis, Pfizer, Takeda, and Roche. A.P.K. reports research funding from AstraZeneca, Bristol Myers Squibb (BMS), Roche/Genentech, Janssen, and AbbVie; serves as a consultant or adviser for AstraZeneca, BMS, Roche/Genentech, Janssen, AbbVie, and LAVA; and other fundings from Janssen, LAVA, AbbVie, and AstraZeneca. P.G. reports research funding from AbbVie, AstraZeneca, Janssen, and BMS; and honoraria from AbbVie, AstraZeneca, BeiGene, Janssen, BMS, Merck Sharp & Dohme, Loxo Oncology/Lilly, and Roche. J.C.B. reports research funding from Zencor and Pharmacyclics; holds a consultant or advisory role for Janssen, Novartis, Syndax, Newave, AstraZeneca, Kura, Vincerx Pharma, Trillium, and AbbVie; and stock ownership in Vincerx Pharma. J.F.S. reports research funding from AbbVie, Celgene, Janssen, and Roche; serves as a consultant or adviser for AbbVie, AstraZeneca, Celgene, Genentech, Genor Biopharma, Gilead, Janssen, MorphoSys, Roche, Sunesis, and TG Therapeutics; and other fundings from AbbVie, Celgene, Roche, and TG Therapeutics. G.D.J. reports employment and stock ownership in AstraZeneca. R.L. and S.R. report employment in AstraZeneca. G.d.B. reports employment in Acerta Pharma BV. V.M. reports employment in AstraZeneca; and stock ownership in AstraZeneca and Gilead Sciences. The remaining authors declare no competing financial interests.
Figures

Comment in
-
A fresh look at covalent BTK inhibitor resistance.Blood. 2024 Sep 5;144(10):1029-1031. doi: 10.1182/blood.2024025237. Blood. 2024. PMID: 39235796 Free PMC article. No abstract available.
References
-
- Gángó A, Alpár D, Galik B, et al. Dissection of subclonal evolution by temporal mutation profiling in chronic lymphocytic leukemia patients treated with ibrutinib. Int J Cancer. 2020;146(1):85–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

