Effectiveness of kangaroo mother care before clinical stabilisation versus standard care among neonates at five hospitals in Uganda (OMWaNA): a parallel-group, individually randomised controlled trial and economic evaluation
- PMID: 38754454
- PMCID: PMC11436264
- DOI: 10.1016/S0140-6736(24)00064-3
Effectiveness of kangaroo mother care before clinical stabilisation versus standard care among neonates at five hospitals in Uganda (OMWaNA): a parallel-group, individually randomised controlled trial and economic evaluation
Erratum in
-
Department of Error.Lancet. 2024 Jun 8;403(10443):2488. doi: 10.1016/S0140-6736(24)01083-3. Epub 2024 May 23. Lancet. 2024. PMID: 38797174 No abstract available.
Abstract
Background: Preterm birth is the leading cause of death in children younger than 5 years worldwide. WHO recommends kangaroo mother care (KMC); however, its effects on mortality in sub-Saharan Africa and its relative costs remain unclear. We aimed to compare the effectiveness, safety, costs, and cost-effectiveness of KMC initiated before clinical stabilisation versus standard care in neonates weighing up to 2000 g.
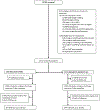
Methods: We conducted a parallel-group, individually randomised controlled trial in five hospitals across Uganda. Singleton or twin neonates aged younger than 48 h weighing 700-2000 g without life-threatening clinical instability were eligible for inclusion. We randomly assigned (1:1) neonates to either KMC initiated before stabilisation (intervention group) or standard care (control group) via a computer-generated random allocation sequence with permuted blocks of varying sizes, stratified by birthweight and recruitment site. Parents, caregivers, and health-care workers were unmasked to treatment allocation; however, the independent statistician who conducted the analyses was masked. After randomisation, neonates in the intervention group were placed prone and skin-to-skin on the caregiver's chest, secured with a KMC wrap. Neonates in the control group were cared for in an incubator or radiant heater, as per hospital practice; KMC was not initiated until stability criteria were met. The primary outcome was all-cause neonatal mortality at 7 days, analysed by intention to treat. The economic evaluation assessed incremental costs and cost-effectiveness from a disaggregated societal perspective. This trial is registered with ClinicalTrials.gov, NCT02811432.
Findings: Between Oct 9, 2019, and July 31, 2022, 2221 neonates were randomly assigned: 1110 (50·0%) neonates to the intervention group and 1111 (50·0%) neonates to the control group. From randomisation to age 7 days, 81 (7·5%) of 1083 neonates in the intervention group and 83 (7·5%) of 1102 neonates in the control group died (adjusted relative risk [RR] 0·97 [95% CI 0·74-1·28]; p=0·85). From randomisation to 28 days, 119 (11·3%) of 1051 neonates in the intervention group and 134 (12·8%) of 1049 neonates in the control group died (RR 0·88 [0·71-1·09]; p=0·23). Even if policy makers place no value on averting neonatal deaths, the intervention would have 97% probability from the provider perspective and 84% probability from the societal perspective of being more cost-effective than standard care.
Interpretation: KMC initiated before stabilisation did not reduce early neonatal mortality; however, it was cost-effective from the societal and provider perspectives compared with standard care. Additional investment in neonatal care is needed for increased impact, particularly in sub-Saharan Africa.
Funding: Joint Global Health Trials scheme of the Department of Health and Social Care, Foreign, Commonwealth and Development Office, UKRI Medical Research Council, and Wellcome Trust; Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

Comment in
-
The road ahead for immediate kangaroo mother care in resource-constrained health systems.Lancet. 2024 Jun 8;403(10443):2459-2461. doi: 10.1016/S0140-6736(24)00268-X. Epub 2024 May 13. Lancet. 2024. PMID: 38754456 No abstract available.
References
-
- United Nations Inter-agency Group for Child Mortality Estimation. Levels & trends in child mortality: report 2023. New York, NY: United Nations Children’s Fund, 2024. https://data.unicef.org/wp-content/uploads/2024/03/UNICEF-2023-Child-Mor... (accessed March 19, 2024).
-
- Oza S, Cousens SN, Lawn JE. Estimation of daily risk of neonatal death, including the day of birth, in 186 countries in 2013: a vital-registration and modelling-based study. Lancet Glob Health 2014; 2: e635–44. - PubMed
-
- Lawn JE, Ohuma EO, Bradley E, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet 2023; 401: 1707–19. - PubMed
-
- WHO. Born too soon: decade of action on preterm birth. 2023. https://www.who.int/publications/i/item/9789240073890 (accessed Dec 4, 2023).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

