Perfluorooctanesulfonic acid exposure leads to downregulation of 3-hydroxy-3-methylglutaryl-CoA synthase 2 expression and upregulation of markers associated with intestinal carcinogenesis in mouse intestinal tissues
- PMID: 38754493
- PMCID: PMC11157449
- DOI: 10.1016/j.chemosphere.2024.142332
Perfluorooctanesulfonic acid exposure leads to downregulation of 3-hydroxy-3-methylglutaryl-CoA synthase 2 expression and upregulation of markers associated with intestinal carcinogenesis in mouse intestinal tissues
Abstract
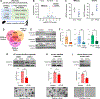
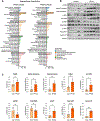
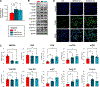
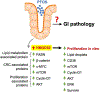
Perfluorooctanesulfonic acid (PFOS) is a widely recognized environment pollutant known for its high bioaccumulation potential and a long elimination half-life. Several studies have shown that PFOS can alter multiple biological pathways and negatively affect human health. Considering the direct exposure to the gastrointestinal (GI) tract to environmental pollutants, PFOS can potentially disrupt intestinal homeostasis. However, there is limited knowledge about the effect of PFOS exposure on normal intestinal tissues, and its contribution to GI-associated diseases remains to be determined. In this study, we examined the effect of PFOS exposure on the gene expression profile of intestinal tissues of C57BL/6 mice using RNAseq analysis. We found that PFOS exposure in drinking water significantly downregulates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2), a rate-limiting ketogenic enzyme, in intestinal tissues of mice. We found that diets containing the soluble fibers inulin and pectin, which are known to be protective against PFOS exposure, were ineffective in reversing the downregulation of HMGCS2 expression in vivo. Analysis of intestinal tissues also demonstrated that PFOS exposure leads to upregulation of proteins implicated in colorectal carcinogenesis, including β-catenin, c-MYC, mTOR and FASN. Consistent with the in vivo results, PFOS exposure leads to downregulation of HMGCS2 in mouse and human normal intestinal organoids in vitro. Furthermore, we show that shRNA-mediated knockdown of HMGCS2 in a human normal intestinal cell line resulted in increased cell proliferation and upregulation of key proliferation-associated proteins such as cyclin D, survivin, ERK1/2 and AKT, along with an increase in lipid accumulation. In summary, our results suggest that PFOS exposure may contribute to pathological changes in normal intestinal cells via downregulation of HMGCS2 expression and upregulation of pro-carcinogenic signaling pathways that may increase the risk of colorectal cancer development.
Keywords: Colorectal carcinogenesis; Environmental pollutants; Gastrointestinal tract; HMGCS2; Ketogenesis; Perfluorooctanesulfonic acid.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Alqurashi N, Gopalan V, Smith RA, Lam AK, 2013. Clinical impacts of mammalian target of rapamycin expression in human colorectal cancers. Hum Pathol 44, 2089–2096. - PubMed
-
- Camarero N, Mascaro C, Mayordomo C, Vilardell F, Haro D, Marrero PF, 2006. Ketogenic HMGCS2 Is a c-Myc target gene expressed in differentiated cells of human colonic epithelium and down-regulated in colon cancer. Mol Cancer Res 4, 645–653. - PubMed
-
- Caron A, Richard D, Laplante M, 2015. The Roles of mTOR Complexes in Lipid Metabolism. Annu Rev Nutr 35, 321–348. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

