Safety and biological outcomes following a phase 1 trial of GD2-specific CAR-T cells in patients with GD2-positive metastatic melanoma and other solid cancers
- PMID: 38754916
- PMCID: PMC11097842
- DOI: 10.1136/jitc-2023-008659
Safety and biological outcomes following a phase 1 trial of GD2-specific CAR-T cells in patients with GD2-positive metastatic melanoma and other solid cancers
Abstract
Background: Chimeric antigen receptor (CAR) T cell therapies specific for the CD19 and B-cell maturation antigen have become an approved standard of care worldwide for relapsed and refractory B-cell malignancies. If CAR-T cell therapy for non-hematological malignancies is to achieve the same stage of clinical development, then iterative early-phase clinical testing can add value to the clinical development process for evaluating CAR-T cell products containing different CAR designs and manufactured under differing conditions.
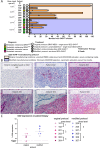
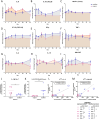
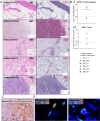
Methods: We conducted a phase 1 trial of third-generation GD2-specific CAR-T cell therapy, which has previously been tested in neuroblastoma patients. In this study, the GD2-CAR-T therapy was evaluated for the first time in metastatic melanoma patients in combination with BRAF/MEK inhibitor therapy, and as a monotherapy in patients with colorectal cancer and a patient with fibromyxoid sarcoma. Feasibility and safety were determined and persistence studies, multiplex cytokine arrays on sera and detailed immune phenotyping of the original CAR-T products, the circulating CAR-T cells, and, in select patients, the tumor-infiltrating CAR-T cells were performed.
Results: We demonstrate the feasibility of manufacturing CAR-T products at point of care for patients with solid cancer and show that a single intravenous infusion was well tolerated with no dose-limiting toxicities or severe adverse events. In addition, we note significant improvements in CAR-T cell immune phenotype, and expansion when a modified manufacturing procedure was adopted for the latter 6 patients recruited to this 12-patient trial. We also show evidence of CAR-T cell-mediated immune activity and in some patients expanded subsets of circulating myeloid cells after CAR-T cell therapy.
Conclusions: This is the first report of third-generation GD2-targeting CAR-T cells in patients with metastatic melanoma and other solid cancers such as colorectal cancer, showing feasibility, safety and immune activity, but limited clinical effect.
Trial registration number: ACTRN12613000198729.
Keywords: Chimeric antigen receptor - CAR; Combination therapy; Solid tumor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- Wikstrand CJ, Fredman P, Svennerholm L, et al. . Detection of glioma-associated Gangliosides GM2, GD2, GD3, 3'-IsoLM1 3',6'-IsoLD1 in central nervous system tumors in vitro and in vivo using EPITOPE-defined Monoclonal antibodies. Prog Brain Res 1994;101:213–23. 10.1016/s0079-6123(08)61951-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
