Granzyme B PET Imaging for Assessment of Disease Activity in Inflammatory Bowel Disease
- PMID: 38754959
- PMCID: PMC11218731
- DOI: 10.2967/jnumed.123.267344
Granzyme B PET Imaging for Assessment of Disease Activity in Inflammatory Bowel Disease
Abstract
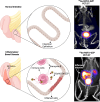
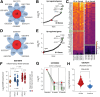
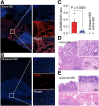
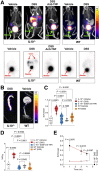
Developing a noninvasive imaging method to detect immune system activation with a high temporal resolution is key to improving inflammatory bowel disease (IBD) management. In this study, granzyme B (GZMB), typically released from cytotoxic T and natural killer cells, was targeted using PET with 68Ga-NOTA-GZP (where GZP is β-Ala-Gly-Gly-Ile-Glu-Phe-Asp-CHO) to detect early intestinal inflammation in murine models of colitis. Methods: Bioinformatic analysis was used to assess the potential of GZMB as a biomarker for detecting IBD and predicting response to treatment. Human active and quiescent Crohn disease and ulcerative colitis tissues were stained for GZMB. We used IL-10-/- mice treated with dextran sulfate sodium (DSS) as an IBD model, wild-type C57BL/6J mice as a control, and anti-tumor necrosis factor as therapy. We used a murine GZMB-binding peptide conjugated to a NOTA chelator (NOTA-GZP) labeled with 68Ga as the PET tracer. PET imaging was conducted at 1, 3, and 4 wk after colitis induction to evaluate temporal changes. Results: Bioinformatic analysis showed that GZMB gene expression is significantly upregulated in human ulcerative colitis and Crohn disease compared with the noninflamed bowel by 2.98-fold and 1.92-fold, respectively; its expression is lower by 2.16-fold in treatment responders than in nonresponders. Immunofluorescence staining of human tissues demonstrated a significantly higher GZMB in patients with active than with quiescent IBD (P = 0.032).68Ga-NOTA-GZP PET imaging showed significantly increased bowel uptake in IL-10-/- mice with DSS-induced colitis compared with vehicle-treated IL-10-/- mice (SUVmean, 0.75 vs. 0.24; P < 0.001) and both vehicle- and DSS-treated wild-type mice (SUVmean, 0.26 and 0.37; P < 0.001). In the IL-10-/- DSS-induced colitis model, the bowel PET probe uptake decreased in response to treatment with tumor necrosis factor-α (SUVmean, 0.32; P < 0.001). There was a 4-fold increase in colonic uptake of 68Ga-NOTA-GZP in the colitis model compared with the control 1 wk after colitis induction. The uptake gradually decreased to approximately 2-fold by 4 wk after IBD induction; however, the inflamed bowel uptake remained significantly higher than control at all time points (week 4 SUVmean, 0.23 vs. 0.08; P = 0.001). Conclusion: GZMB is a promising biomarker to detect active IBD and predict response to treatment. This study provides compelling evidence to translate GZMB PET for imaging IBD activity in clinical settings.
Keywords: 68Ga-NOTA-GZP; PET imaging; colitis; granzyme B; inflammatory bowel disease.
© 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
Similar articles
-
Granzyme B PET Imaging Predicts Response to Immunotherapy in a Diet-Induced Obesity Model of Breast Cancer.J Nucl Med. 2025 Jul 1;66(7):1039-1045. doi: 10.2967/jnumed.124.268938. J Nucl Med. 2025. PMID: 40441892 Free PMC article.
-
Translocator protein facilitates neutrophil-mediated mucosal inflammation in inflammatory bowel diseases.World J Gastroenterol. 2025 Jul 21;31(27):109239. doi: 10.3748/wjg.v31.i27.109239. World J Gastroenterol. 2025. PMID: 40741102 Free PMC article.
-
ImmunoPET imaging of c-Met using a nanobody-based tracer [68Ga]Ga-NOTA-PFCM01 in pancreatic ductal adenocarcinoma models and non-human primates.Eur J Nucl Med Mol Imaging. 2025 Jul 9. doi: 10.1007/s00259-025-07441-6. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40632219
-
Vitamin D for the treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2023 Oct 2;10(10):CD011806. doi: 10.1002/14651858.CD011806.pub2. Cochrane Database Syst Rev. 2023. PMID: 37781953 Free PMC article.
-
Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD000279. doi: 10.1002/14651858.CD000279.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Sep 18;9:CD000279. doi: 10.1002/14651858.CD000279.pub4. PMID: 16625534 Updated.
Cited by
-
Biological characteristics, immune infiltration and drug prediction of PANoptosis related genes and possible regulatory mechanisms in inflammatory bowel disease.Sci Rep. 2025 Jan 15;15(1):2033. doi: 10.1038/s41598-024-84911-1. Sci Rep. 2025. PMID: 39814753 Free PMC article.
-
Molecular imaging using (nano)probes: cutting-edge developments and clinical challenges in diagnostics.RSC Adv. 2025 Jul 14;15(30):24696-24725. doi: 10.1039/d5ra03927d. eCollection 2025 Jul 10. RSC Adv. 2025. PMID: 40661211 Free PMC article. Review.
-
Anti-integrin αvβ6 IgG antibody as a diagnostic and prognostic marker in ulcerative colitis: A cross-sectional and longitudinal study defining a specific disease phenotype.J Crohns Colitis. 2025 May 8;19(5):jjaf062. doi: 10.1093/ecco-jcc/jjaf062. J Crohns Colitis. 2025. PMID: 40251889 Free PMC article.
-
Approaches to Imaging Immune Activation Using PET.J Nucl Med. 2025 Jun 2;66(6):839-847. doi: 10.2967/jnumed.124.268289. J Nucl Med. 2025. PMID: 40306967 Review.
-
CD45-targeted PET enables the visualization of inflammatory conditions.Am J Nucl Med Mol Imaging. 2025 Jun 25;15(3):124-129. doi: 10.62347/VFWR2835. eCollection 2025. Am J Nucl Med Mol Imaging. 2025. PMID: 40688532 Free PMC article.
References
-
- Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn’s disease. Autoimmun Rev. 2014;13:467–471. - PubMed
-
- Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev. 2014;13:463–466. - PubMed
-
- Lightner AL, Moncrief SB, Smyrk TC, et al. . Long-standing Crohn’s disease and its implication on anal squamous cell cancer management. Int J Colorectal Dis. 2017;32:661–666. - PubMed
-
- Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–410. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
