Fermented Milk Containing Lacticaseibacillus rhamnosus SNU50430 Modulates Immune Responses and Gut Microbiota in Antibiotic-Treated Mice
- PMID: 38755001
- PMCID: PMC11239404
- DOI: 10.4014/jmb.2401.01012
Fermented Milk Containing Lacticaseibacillus rhamnosus SNU50430 Modulates Immune Responses and Gut Microbiota in Antibiotic-Treated Mice
Abstract
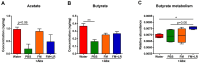
Antibiotics are used to control infectious diseases. However, adverse effects of antibiotics, such as devastation of the gut microbiota and enhancement of the inflammatory response, have been reported. Health benefits of fermented milk are established and can be enhanced by the addition of probiotic strains. In this study, we evaluated effects of fermented milk containing Lacticaseibacillus rhamnosus (L. rhamnosus) SNUG50430 in a mouse model with antibiotic treatment. Fermented milk containing 2 × 105 colony-forming units of L. rhamnosus SNUG50430 was administered to six week-old female BALB/c mice for 1 week. Interleukin (IL)-10 levels in colon samples were significantly increased (P < 0.05) compared to water-treated mice, whereas interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) were decreased, of mice treated with fermented milk containing L. rhamnosus SNUG50430-antibiotics-treated (FM+LR+Abx-treated) mice. Phylum Firmicutes composition in the gut was restored and the relative abundances of several bacteria, including the genera Coprococcus and Lactobacillus, were increased in FM+LR+Abx-treated mice compared to PBS+Abx-treated mice. Interestingly, abundances of genus Coprococcus and Lactobacillus were positively correlated with IL-5 and IL-10 levels (P < 0.05) in colon samples and negative correlated with IFN-γ and TNF-α levels in serum samples (P < 0.001). Acetate and butyrate were increased in mice with fermented milk and fecal microbiota of FM+LR+Abx-treated mice were highly enriched with butyrate metabolism pathway compared to water-treated mice (P < 0.05). Thus, fermented milk containing L. rhamnosus SNUG50430 was shown to ameliorate adverse health effects caused by antibiotics through modulating immune responses and the gut microbiota.
Keywords: Antibiotic; Lacticaseibacillus rhamnosus; fermented milk; gut microbiota; immunomodulation; probiotic.
Conflict of interest statement
G.K. is the founder of KoBioLabs, Inc., and S.P. is an employee by KoBioLabs, Inc and weBiom Inc. S.E.J. and I.C. are employees by hy Co., Ltd. Remaining authors, S.Y., C.L., and W.-K.K., have no financial conflicts of interest to declare.
Figures






Similar articles
-
Regulation of gut microbiota and serum neurotransmitters in mice by Streptococcus thermophilus GA8- and Lacticaseibacillus rhamnosus HAO9-fermented milk containing high levels of gamma-aminobutyric acid.J Sci Food Agric. 2024 Oct;104(13):8050-8058. doi: 10.1002/jsfa.13634. Epub 2024 Jun 3. J Sci Food Agric. 2024. PMID: 38828862
-
Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation.BMC Microbiol. 2021 May 13;21(1):146. doi: 10.1186/s12866-021-02192-4. BMC Microbiol. 2021. PMID: 33985438 Free PMC article.
-
Lactobacillus rhamnosus and Bifidobacterium longum alleviate colitis and cognitive impairment in mice by regulating IFN-γ to IL-10 and TNF-α to IL-10 expression ratios.Sci Rep. 2021 Oct 19;11(1):20659. doi: 10.1038/s41598-021-00096-x. Sci Rep. 2021. PMID: 34667205 Free PMC article.
-
Impact of Fermented Milk On Gut Microbiota And Human Health: A Comprehensive Review.Curr Microbiol. 2025 Jan 31;82(3):107. doi: 10.1007/s00284-025-04061-z. Curr Microbiol. 2025. PMID: 39888432 Review.
-
Lactobacillus rhamnosus: An emerging probiotic with therapeutic potential for depression.Pharmacol Res. 2025 Jan;211:107541. doi: 10.1016/j.phrs.2024.107541. Epub 2024 Dec 7. Pharmacol Res. 2025. PMID: 39653301 Review.
Cited by
-
Substitutive Effects of Milk vs. Vegetable Milk on the Human Gut Microbiota and Implications for Human Health.Nutrients. 2024 Sep 14;16(18):3108. doi: 10.3390/nu16183108. Nutrients. 2024. PMID: 39339708 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

