Achieving osteoporosis treat-to-target goals with teriparatide or alendronate: sub-analysis of Japanese Osteoporosis Intervention Trial-05 (JOINT-05)
- PMID: 38755328
- PMCID: PMC11147828
- DOI: 10.1007/s00774-024-01515-5
Achieving osteoporosis treat-to-target goals with teriparatide or alendronate: sub-analysis of Japanese Osteoporosis Intervention Trial-05 (JOINT-05)
Abstract
Introduction: The purpose of this study was to evaluate whether bone mineral density (BMD) ≥ -2.5 SD could be used as the treat-to-target (T2T) goal when treating osteoporosis with teriparatide (TPTD) and alendronate (ALN), and to investigate the relationship with incident vertebral fracture by re-analyzing data from a randomized, controlled trial (JOINT-05) involving postmenopausal Japanese women at high fracture risk.
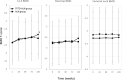
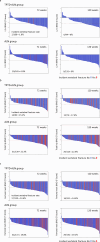
Materials and methods: Participants received sequential therapy with once-weekly TPTD for 72 weeks, followed by ALN for 48 weeks (TPTD-ALN group) or ALN monotherapy for 120 weeks (ALN group). BMDs were measured at the lumbar spine (L2-4), total hip, and femoral neck at 0, 24, 48, 72, and 120 weeks by dual-energy X-ray absorptiometry. The T2T goal was BMD ≥ -2.5 SD, and the endpoint was the proportion of participants with baseline BMD < -2.5 SD in three measurement sites achieving BMD ≥ -2.5 SD.
Results: A total of 559 participants were selected. BMD ≥ -2.5 SD at 120 weeks in the L2-4, total hip, and femoral neck sites was achieved in 20.5%, 23.1%, and 5.9%, respectively, in the TPTD-ALN group and 22.2%, 11.7%, and 7.3%, respectively, in the ALN group. Incident vertebral fractures occurred in areas of both lower and high BMD.
Conclusion: During the 1.5-year treatment period, more than 20% of participants achieved BMD ≥ -2.5 SD as a T2T goal at L2-4. Since the achievement level differed depending on the BMD measurement site, the appropriate site should be selected according to the baseline BMD level.
Keywords: Bone mineral density; Measurement site; Osteoporosis; Teriparatide; Treat-to-target.
© 2024. The Author(s).
Conflict of interest statement
Dr. Hagino received lecture fees or grants outside the submitted work from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Mochida Pharma Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd. Dr. Tanaka has received lecture fees from the Research Institute of Healthcare Data Science. He has received consultation and outsourcing fees from Eli Lilly and Company, Welby, Daiichi Sankyo Company, Limited, Janssen Pharmaceutical K.K., Satt, and the Public Health Research Foundation. He has received research grants from the Japan Agency for Medical Research and Development, the Japanese Ministry of Health Labour and Welfare, the Japanese Ministry of Education, Science, and Technology, and Novo Nordisk. He engaged in a research project of the Japan Agency for Medical Research and Development. Dr. Soen has received consulting fees, speaking fees, and/or honoraria from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mochida Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd. Dr. Kuroda and Dr. Mori declare no competing interests.
Figures
Similar articles
-
Association between renal function and fracture incidence during treatment with teriparatide or alendronate: an exploratory subgroup analysis of the Japanese Osteoporosis Intervention Trial-05.Osteoporos Int. 2024 Dec;35(12):2175-2182. doi: 10.1007/s00198-024-07260-9. Epub 2024 Sep 30. Osteoporos Int. 2024. PMID: 39343826 Free PMC article. Clinical Trial.
-
Sequential treatment of teriparatide and alendronate versus alendronate alone for elevation of bone mineral density and prevention of refracture after percutaneous vertebroplasty in osteoporosis: a prospective study.Aging Clin Exp Res. 2023 Mar;35(3):531-539. doi: 10.1007/s40520-023-02342-w. Epub 2023 Jan 28. Aging Clin Exp Res. 2023. PMID: 36708462 Free PMC article.
-
Overlapping and continued alendronate or raloxifene administration in patients on teriparatide: effects on areal and volumetric bone mineral density--the CONFORS Study.J Bone Miner Res. 2014 Aug;29(8):1777-85. doi: 10.1002/jbmr.2216. J Bone Miner Res. 2014. PMID: 24619763 Clinical Trial.
-
Effects of teriparatide versus alendronate for treatment of postmenopausal osteoporosis: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2017 May;96(21):e6970. doi: 10.1097/MD.0000000000006970. Medicine (Baltimore). 2017. PMID: 28538396 Free PMC article. Review.
-
Is abaloparatide more efficacious on increasing bone mineral density than teriparatide for women with postmenopausal osteoporosis? An updated meta-analysis.J Orthop Surg Res. 2023 Feb 17;18(1):116. doi: 10.1186/s13018-023-03595-x. J Orthop Surg Res. 2023. PMID: 36797767 Free PMC article. Review.
Cited by
-
Clinical Efficacy and Safety of Teriparatide Versus Alendronate in Postmenopausal Osteoporosis: A Systematic Review of Randomized Controlled Trials.Cureus. 2024 Nov 5;16(11):e73068. doi: 10.7759/cureus.73068. eCollection 2024 Nov. Cureus. 2024. PMID: 39640163 Free PMC article. Review.
References
-
- Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pickard L, Kennedy CC, Prior JC, Olszynski WP, Davison KS, Goltzman D, Thabane L, Gafni A, Papadimitropoulos EA, Brown JP, Josse RG, Hanley DA, Adachi JD. Relation between fractures and mortality: results from the Canadian multicentre osteoporosis study. CMAJ. 2009;181:265–271. doi: 10.1503/cmaj.081720. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

