Effectiveness and tolerability of eptinezumab in treating patients with migraine resistant to conventional preventive medications and CGRP (receptor) antibodies: a multicentre retrospective real-world analysis from Germany
- PMID: 38755541
- PMCID: PMC11097519
- DOI: 10.1186/s10194-024-01788-1
Effectiveness and tolerability of eptinezumab in treating patients with migraine resistant to conventional preventive medications and CGRP (receptor) antibodies: a multicentre retrospective real-world analysis from Germany
Abstract
Background: Eptinezumab is a monoclonal antibody that targets calcitonin gene-related peptide (CGRP mAb) and is used for migraine prophylaxis. Efficacy data are mainly from clinical trials, real-world data are hardly available yet. Reimbursement policy in Germany leads to eptinezumab mainly being used in patients having failed pre-treatment with other CGRP mAb. To date, it is unclear whether eptinezumab is efficacious and well tolerated in this population and how the treatment response differs from patients who are naive to CGRP mAbs.
Methods: We analysed clinical routine data of 79 patients (episodic migraine (EM): n = 19; chronic migraine (CM): n = 60) from four different centres in Germany. All patients were treated with eptinezumab (100mg). Differences in monthly headache (MHD), migraine (MMD) and acute medication days (AMD) after three months were analysed. The correlation of response with the number of CGRP mAb failures was evaluated. Significance level has been corrected (alpha = 0.017).
Results: After three months MHD, MMD and AMD were significantly reduced. In EM, the median reduction for MHD was 4.0 days (IQR: -6.5 to -1.0; p = 0.001), for MMD 3.0 days (IQR: -5.5 to -1.5; p < 0.001) and for AMD 2.0 days (IQR: -5.0 to -0.5; p = 0.006). In CM, median reduction of MHD was 4 days (IQR: -8.0 to 0.0; p < 0.001), 3.0 days (IQR: -6.0 to-1.0; p < 0.001) for MMD and 1.0 day (IQR: -5.0 to 0.0; p < 0.001) for AMD. All patients were resistant to conventional preventive therapies and most to CGRP mAbs. Fourteen patients had never received a CGRP mAb and 65 patients had received at least one mAb without sufficient effectiveness and/or intolerability (one: n = 20, two: n = 28, three: n = 17). There was a significant association between the number of prior therapies and the 30% MHD responder rate (none: 78.6%, one: 45.0%, two: 32.1%, three: 23.5%, p = 0.010). Regarding tolerability, 10.4% (8/77) reported mild side effects.
Conclusions: The effectiveness of eptinezumab is significantly reduced in patients who have not previously responded to other CGRP mAbs. However, limitations such as the retrospective nature of the analysis, the small sample size and the short treatment period with only the lower dose of eptinezumab must be considered when interpreting the results.
Keywords: Antibody switch; Chronic migraine; Drug resistant; Episodic migraine.
© 2024. The Author(s).
Conflict of interest statement
PB received honoraria for speaking engagements from Novartis, Eli Lilly, Teva, Allergan Pharma/AbbVie and Lundbeck, for advisory boards from Teva and received scientific support from Novartis.
DH has received scientific support and/or honoraria from Biogen, Novartis, Eli Lilly, Sanofi-Aventis, Teva, Allergan, Hormosan.
RF received honoraria and travel fees, a consulting or advisory role to declare from Teva, Eli Lilly, Allergan Pharma/AbbVie, Ipsen, Lundbeck and Novartis.
AG has received non-financial support and personal fees for talks and adboards, non-personal support for participation in clinical trials from Allergan, Eli Lilly, Hormosan, Teva Pharmaceutical, Grunenthal, Mundipharma, Esanum, DGS. AG reports personal fees from Eli Lilly, Novartis and Teva Pharmaceutical.
CK has received honoraria, a consulting or advisory role to declare from Novartis and Teva.
MN received travel fees from Licher MT.
SN received financial support for consulting and speaking engagements from Allergan, Hormosan, Lilly, Lundbeck, Novartis and Teva.
AS has received travel fees from Teva and honoraria from Novartis (advisory board).
JB, MB, PW declare that there is no conflict of interest.
Figures

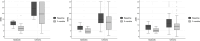

References
-
- Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy–2) study. J Headache Pain. 2020;21:120. doi: 10.1186/s10194-020-01186-3. - DOI - PMC - PubMed
-
- Ashina M, Lanteri-Minet M, Pozo-Rosich P, et al. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2022;21:597–607. doi: 10.1016/S1474-4422(22)00185-5. - DOI - PubMed
-
- Ashina M, Lanteri-Minet M, Ettrup A, et al. Efficacy and safety of eptinezumab for migraine prevention in patients with prior preventive treatment failures: subgroup analysis of the randomized, placebo-controlled DELIVER study. Cephalalgia. 2023;43:3331024231170807. doi: 10.1177/03331024231170807. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

