Insights for clinical management from the real-life data of the centralized West of Scotland biliary cancer clinic
- PMID: 38755562
- PMCID: PMC11097428
- DOI: 10.1186/s12885-024-12279-6
Insights for clinical management from the real-life data of the centralized West of Scotland biliary cancer clinic
Abstract
Background: With the increasing of novel therapeutics for the treatment of Biliary Tract Cancers (BTC), and the need to assess their socio-economic impacts for national licence approvals, it is as important as ever to have real-life data in national populations.
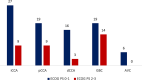
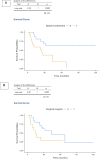
Methods and results: We performed an audit of the first 2 year-activity (Sep 2019-Sep 2021) of the centralized West-of-Scotland-BTC clinic. 122 patients accessed the service, including 68% with cholangiocarcinoma (CCA), 27% with gallbladder cancer (GBC), and 5% with ampulla of Vater carcinoma with biliary phenotype (AVC). Median age at diagnosis was 66 (28-84), with 30% of newly diagnosed patients being younger than 60 years-old. Thirty-five cases (29%) underwent surgery, followed by adjuvant-chemotherapy in 66%. 60% had recurrent disease (80% with distant relapse). Sixty-four patients (58%) started first-line Systemic-AntiCancer-Treatment (SACT). Of these, 37% received second line SACT, the majority of which had iCCA and GBC. Thirty-% of those who progressed received third line SACT.
Conclusions: About 30% of BTC were eligible for curative surgery. Fifty-eight and twenty% of the overall cohort of advanced BTC patients received first and second line SACT. Our data suggest that reflex genomic profiling may not be cost-effective until molecularly driven strategies are limited to second line setting.
Keywords: Biliary cancer; Chemotherapy; Cholangiocarcinoma; Genomic profiling; Scotland; Systemic treatment.
© 2024. The Author(s).
Conflict of interest statement
Competing Interests: CB received honoraria as speaker (Astrazeneca, Incyte, Servier) and consultant (Incyte, Servier, Boehringer Ingelheim, Astrazeneca), received research funds (Avacta, Medannex, Servier) and her spouse is an employee of Astrazeneca. VZ, TN, RC, GA, OP and JM have no conflict of interest.
Figures
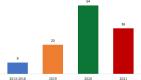



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

