MicroRNA profiling in women with migraine: effects of CGRP-targeting treatment
- PMID: 38755568
- PMCID: PMC11100066
- DOI: 10.1186/s10194-024-01787-2
MicroRNA profiling in women with migraine: effects of CGRP-targeting treatment
Abstract
Background: Migraine lacks biomarkers that can trace the biological pathways of the disease and predict the effectiveness of treatments. Monoclonal antibodies targeting calcitonin gene-related peptide pathway - including erenumab - offer the opportunity of investigating potential migraine biomarkers due to their specific mechanism of action in preventing both episodic (EM) and chronic (CM) migraine. Our study aims at evaluating the expression levels of circulating microRNAs (miRNAs) according to migraine type, before and after treatment with erenumab and based on treatment response, in order to identify miRNAs with potential role as epigenetic biomarkers.
Methods: The study included women aged 25-50 years with EM or CM treated with erenumab according to clinical indications. MiRNAs expression levels were assessed before (baseline) and after a 16-week treatment with erenumab, 140 mg every four weeks (post-treatment). An extensive miRNAs profiling was performed by qRT-PCR in small, pooled groups of ≤ 8 women each, classified according to migraine frequency (EM and CM) and the degree of response to erenumab. The expression levels of selected miRNAs were also validated using single miRNA assays in each woman with EM and CM.
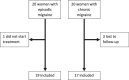
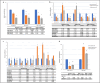
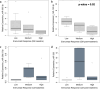
Results: During the study, 36 women with migraine (19 with EM and 17 with CM) out of 40 who were initially screened, performed the assessment of miRNA expression at baseline and post-treatment, Erenumab treatment significantly improved migraine burden in both EM and CM. MiRNA profiling revealed differential expression levels of a wide set of miRNAs (hsa-let-7d-3p, hsa-miR-106b-3p, hsa-miR-122-5p, hsa-miR-143-3p, hsa-miR-144-3p, hsa-miR-16-5p, hsa-miR-181a-5p, hsa-miR-221-3p, hsa-miR-25-3p, hsa-miR-29b-2-5p, hsa-miR-326, miR-363-3p, hsa-miR-424-5p, hsa-miR-485-3p, hsa-miR-532-5p, hsa-miR-543, hsa-miR-629-5p, hsa-miR-660-5p, hsa-miR-92a-3p) depending on treatment response. Among them, single miRNA assays confirmed the progressive decrease of hsa-miR-143-3p expression levels in relation to increasing response to erenumab in women with EM (7 with low, 6 with medium, and 6 with high response; p = 0.02). Additionally, single assays showed higher hsa-miR-34a-5p and hsa-miR-382-5p expression levels at baseline in women with CM compared with those with EM (p = 0.0002 and p = 0.0007, respectively), as well as their expression level decrease in women with CM from baseline to follow-up (p = 0.04 and p = 0.02, respectively).
Conclusions: Our study suggests that targeting the CGRP pathway in migraine changes the expression levels of certain miRNAs. These miRNA levels are linked to the levels of response to CGRP receptor blockage. Future research challenges include assigning specific functions to the modulated miRNAs to unravel pathways modulated by the disease and the treatment.
Trial registration: The study was registered in clinicaltrials.gov with code NCT04659226 and in the Novartis database with code CAMG334AIT05T.
Keywords: Biomarkers; Epigenetics; Erenumab; MicroRNA; Migraine prevention; Monoclonal antibodies.
© 2024. The Author(s).
Conflict of interest statement
SS reports grants, personal fees and/or non-financial support from Allergan, Novartis, Teva, Eli Lilly, Astrazeneca, Medscape, Pfizer, Bayer, Medtronic, Starmed, Bristol-Myers-Squibb, and Daiichi-Sankyo. RO reports personal fees from AbbVie, Eli Lilly, Novartis, Pfizer, Teva, and non-financial support from Allergan-AbbVie, Eli Lilly, Novartis, and Teva. All other Authors report no competing interests.
Figures



References
-
- Safiri S, Pourfathi H, Eagan A, Mansournia MA, Khodayari MT, Sullman MJM, Kaufman J, Collins G, Dai H, Bragazzi NL, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. 2022;163(2):e293–e309. doi: 10.1097/j.pain.0000000000002275. - DOI - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

