The effects of grape seed extract supplementation on cardiovascular risk factors, liver enzymes and hepatic steatosis in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study
- PMID: 38755622
- PMCID: PMC11100156
- DOI: 10.1186/s12906-024-04477-3
The effects of grape seed extract supplementation on cardiovascular risk factors, liver enzymes and hepatic steatosis in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study
Abstract
Background: Despite the high antioxidant potential of grape seed extract (GSE), very limited studies have investigated its effect on non-alcoholic fatty liver disease (NAFLD). Therefore, this study was conducted with the aim of investigating the effect of GSE on metabolic factors, blood pressure and steatosis severity in patients with NAFLD.
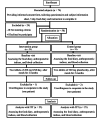
Methods: In this double-blind randomized clinical trial study, 50 NAFLD patients were divided into two groups of 25 participants who were treated with 520 mg/day of GSE or the placebo group for 2 months. The parameters of glycemic, lipid profile, blood pressure and steatohepatitis were measured before and after the intervention.
Results: The GSE group had an average age of 43.52 ± 8.12 years with 15 women and 10 men, while the placebo group had an average age of 44.88 ± 10.14 years with 11 women and 14 men. After 2 months of intervention with GSE, it was observed that insulin, HOMA-IR, TC, TG, LDL-c, ALT, AST, AST/ALT, SBP, DBP and MAP decreased and QUICKi and HDL-c increased significantly (p-value for all < 0.05). Also, before and after adjustment based on baseline, the average changes indicated that the levels of insulin, HOMA-IR, TC, TG, LDL-c, SBP, DBP, MAP in the GSE group decreased more than in the control group (p for all < 0.05). Furthermore, the changes in HDL-c were significantly higher in the GSE group (p < 0.05). The between-groups analysis showed a significant decrease in the HOMA-β and AST before and after adjustment based on baseline levels (p < 0.05). Moreover, the changes in QUICKi after adjustment based on baseline levels were higher in the GSE group than in the control group. Also, between-groups analysis showed that the severity of hepatic steatosis was reduced in the intervention group compared to the placebo group (P = 0.002).
Conclusions: It seems that GSE can be considered one of the appropriate strategies for controlling insulin resistance, hyperlipidemia, hypertension and hepatic steatosis in NAFLD patients.
Trial registration: The clinical trial was registered in the Iranian Clinical Trial Registration Center (IRCT20190731044392N1). https://irct.behdasht.gov.ir/trial/61413 . (The registration date: 30/03/2022).
Keywords: Grape seed extract; Hyperglycemia; Hyperlipidemia; Hypertension; Non-alcoholic fatty liver disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no conflict of interest.
Similar articles
-
Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: a randomised, double-blinded, two-arm, parallel, placebo-controlled trial.Br J Nutr. 2016 Jan 28;115(2):226-38. doi: 10.1017/S0007114515004328. Epub 2015 Nov 16. Br J Nutr. 2016. PMID: 26568249 Clinical Trial.
-
Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: a double-blind randomized placebo-controlled clinical trial.BMC Complement Altern Med. 2019 Mar 12;19(1):59. doi: 10.1186/s12906-019-2465-0. BMC Complement Altern Med. 2019. PMID: 30871514 Free PMC article. Clinical Trial.
-
Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial.Int J Clin Pract. 2021 Nov;75(11):e14852. doi: 10.1111/ijcp.14852. Epub 2021 Sep 18. Int J Clin Pract. 2021. PMID: 34516703 Clinical Trial.
-
Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis.Front Immunol. 2022 Sep 9;13:949746. doi: 10.3389/fimmu.2022.949746. eCollection 2022. Front Immunol. 2022. PMID: 36159792 Free PMC article.
-
Curcumin effects on glycaemic indices, lipid profile, blood pressure, inflammatory markers and anthropometric measurements of non-alcoholic fatty liver disease patients: A systematic review and meta-analysis of randomized clinical trials.Complement Ther Med. 2024 Mar;80:103025. doi: 10.1016/j.ctim.2024.103025. Epub 2024 Jan 15. Complement Ther Med. 2024. PMID: 38232906
References
-
- Hormati A, Shakeri M, Iranikhah A, Afifian M, Sarkeshikian SS. Non-alcoholic fatty liver disease. Govaresh. 2018;23(4):203–12.
-
- Salehisahlabadi A, jadid h The prevalence of non-alcoholic fatty liver disease in Iranian children and adolescents: a systematic review and Meta-analysis. J Sabzevar Univ Med Sci. 2018;25(4):487–94.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous