Extracellular vesicles from senescent mesenchymal stromal cells are defective and cannot prevent osteoarthritis
- PMID: 38755672
- PMCID: PMC11097483
- DOI: 10.1186/s12951-024-02509-1
Extracellular vesicles from senescent mesenchymal stromal cells are defective and cannot prevent osteoarthritis
Erratum in
-
Correction: Extracellular vesicles from senescent mesenchymal stromal cells are defective and cannot prevent osteoarthritis.J Nanobiotechnology. 2024 Jul 16;22(1):418. doi: 10.1186/s12951-024-02655-6. J Nanobiotechnology. 2024. PMID: 39014465 Free PMC article. No abstract available.
Abstract
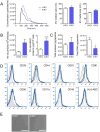
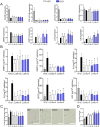
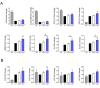
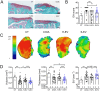
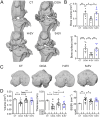
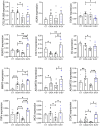
Age is the most important risk factor in degenerative diseases such as osteoarthritis (OA), which is associated with the accumulation of senescent cells in the joints. Here, we aimed to assess the impact of senescence on the therapeutic properties of extracellular vesicles (EVs) from human fat mesenchymal stromal cells (ASCs) in OA. We generated a model of DNA damage-induced senescence in ASCs using etoposide and characterized EVs isolated from their conditioned medium (CM). Senescent ASCs (S-ASCs) produced 3-fold more EVs (S-EVs) with a slightly bigger size and that contain 2-fold less total RNA. Coculture experiments showed that S-ASCs were as efficient as healthy ASCs (H-ASCs) in improving the phenotype of OA chondrocytes cultured in resting conditions but were defective when chondrocytes were proliferating. S-EVs were also impaired in their capacity to polarize synovial macrophages towards an anti-inflammatory phenotype. A differential protein cargo mainly related to inflammation and senescence was detected in S-EVs and H-EVs. Using the collagenase-induced OA model, we found that contrary to H-EVs, S-EVs could not protect mice from cartilage damage and joint calcifications, and were less efficient in protecting subchondral bone degradation. In addition, S-EVs induced a pro-catabolic and pro-inflammatory gene signature in the joints of mice shortly after injection, while H-EVs decreased hypertrophic, catabolic and inflammatory pathways. In conclusion, S-EVs are functionally impaired and cannot protect mice from developing OA.
Keywords: Aging; Extracellular vesicle; Mesenchymal stromal cell; Osteoarthritis; Regenerative medicine; Senescence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, Fini M, Giardino R, Facchini A, Grigolo B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013;15:R22. 10.1186/ar4156 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

