Hyperoside mitigates photoreceptor degeneration in part by targeting cGAS and suppressing DNA-induced microglial activation
- PMID: 38755736
- PMCID: PMC11097432
- DOI: 10.1186/s40478-024-01793-0
Hyperoside mitigates photoreceptor degeneration in part by targeting cGAS and suppressing DNA-induced microglial activation
Abstract
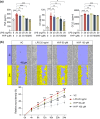
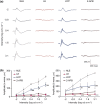
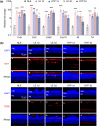
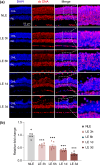
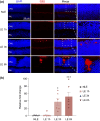
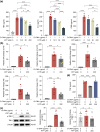
Activated microglia play an important role in driving photoreceptor degeneration-associated neuroinflammation in the retina. Controlling pro-inflammatory activation of microglia holds promise for mitigating the progression of photoreceptor degeneration. Our previous study has demonstrated that pre-light damage treatment of hyperoside, a naturally occurring flavonol glycoside with antioxidant and anti-inflammatory activities, prevents photooxidative stress-induced photoreceptor degeneration and neuroinflammatory responses in the retina. However, the direct impact of hyperoside on microglia-mediated neuroinflammation during photoreceptor degeneration remains unknown. Upon verifying the anti-inflammatory effects of hyperoside in LPS-stimulated BV-2 cells, our results here further demonstrated that post-light damage hyperoside treatment mitigated the loss of photoreceptors and attenuated the functional decline of the retina. Meanwhile, post-light damage hyperoside treatment lowered neuroinflammatory responses and dampened microglial activation in the illuminated retinas. With respect to microglial activation, hyperoside mitigated the pro-inflammatory responses in DNA-stimulated BV-2 cells and lowered DNA-stimulated production of 2'3'-cGAMP in BV-2 cells. Moreover, hyperoside was shown to directly interact with cGAS and suppress the enzymatic activity of cGAS in a cell-free system. In conclusion, the current study suggests for the first time that the DNA sensor cGAS is a direct target of hyperoside. Hyperoside is effective at mitigating DNA-stimulated cGAS-mediated pro-inflammatory activation of microglia, which likely contributes to the therapeutic effects of hyperoside at curtailing neuroinflammation and alleviating neuroinflammation-instigated photoreceptor degeneration.
Keywords: Hyperoside; Microglia activation; Neuroinflammation; Photoreceptor degeneration; cGAS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Nicotinamide Riboside Mitigates Retinal Degeneration by Suppressing Damaged DNA-Stimulated Microglial Activation and STING-Mediated Pyroptosis.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):14. doi: 10.1167/iovs.66.4.14. Invest Ophthalmol Vis Sci. 2025. PMID: 40192637 Free PMC article.
-
Ginsenoside Re Mitigates Photooxidative Stress-Mediated Photoreceptor Degeneration and Retinal Inflammation.J Neuroimmune Pharmacol. 2023 Sep;18(3):397-412. doi: 10.1007/s11481-023-10073-y. Epub 2023 Jun 16. J Neuroimmune Pharmacol. 2023. PMID: 37326907 Free PMC article.
-
Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration.J Neuroinflammation. 2015 Nov 17;12:209. doi: 10.1186/s12974-015-0431-4. J Neuroinflammation. 2015. PMID: 26576678 Free PMC article.
-
Hyperoside protects against oxidative stress-mediated photoreceptor degeneration: therapeutic potentials for photoreceptor degenerative diseases.J Transl Med. 2023 Aug 24;21(1):569. doi: 10.1186/s12967-023-04459-y. J Transl Med. 2023. PMID: 37620913 Free PMC article.
-
The multiple biological activities of hyperoside: from molecular mechanisms to therapeutic perspectives in neoplastic and non-neoplastic diseases.Front Pharmacol. 2025 Mar 3;16:1538601. doi: 10.3389/fphar.2025.1538601. eCollection 2025. Front Pharmacol. 2025. PMID: 40098612 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

