A Gelatin Hydrogel Nonwoven Fabric Combined With Adipose Tissue-Derived Stem Cells Enhances Subcutaneous Islet Engraftment
- PMID: 38756050
- PMCID: PMC11102670
- DOI: 10.1177/09636897241251621
A Gelatin Hydrogel Nonwoven Fabric Combined With Adipose Tissue-Derived Stem Cells Enhances Subcutaneous Islet Engraftment
Abstract
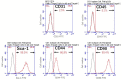
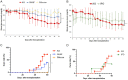
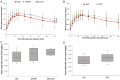
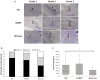
Subcutaneous islet transplantation is a promising treatment for severe diabetes; however, poor engraftment hinders its prevalence. We previously revealed that a gelatin hydrogel nonwoven fabric (GHNF) markedly improved subcutaneous islet engraftment. We herein investigated whether the addition of adipose tissue-derived stem cells (ADSCs) to GHNF affected the outcome. A silicone spacer sandwiched between two GHNFs with (AG group) or without (GHNF group) ADSCs, or a silicone spacer alone (Silicone group) was implanted into the subcutaneous space of healthy mice at 6 weeks before transplantation, then diabetes was induced 7 days before transplantation. Syngeneic islets were transplanted into the pretreated space. Intraportal transplantation (IPO group) was also performed to compare the transplant efficiency. Blood glucose, intraperitoneal glucose tolerance, immunohistochemistry, and inflammatory mediators were evaluated. The results in the subcutaneous transplantation were compared using the Silicone group as a control. The results of the IPO group were also compared with those of the AG group. The AG group showed significantly better blood glucose changes than the Silicone and the IPO groups. The cure rate of AG group (72.7%) was the highest among the groups (GHNF; 40.0%, IPO; 40.0%, Silicone; 0%). The number of vWF-positive vessels in the subcutaneous space of the AG group was significantly higher than that in other groups before transplantation (P < 0.01). Lectin angiography also showed that the same results (P < 0.05). According to the results of the ADSCs tracing, ADSCs did not exist at the transplant site (6 weeks after implantation). The positive rates for laminin and collagen III constructed around the transplanted islets did not differ among groups. Inflammatory mediators were higher in the Silicone group, followed by the AG and GHNF groups. Pretreatment using bioabsorbable scaffolds combined with ADSCs enhanced neovascularization in subcutaneous space, and subcutaneous islet transplantation using GHNF with ADSCs was superior to intraportal islet transplantation.
Keywords: adipose tissue–derived stem cells; extracellular matrix; gelatin hydrogel nonwoven fabrics; islet; neovascularization; subcutaneous transplantation.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare no conflicts of interest in association with the present study, although this study was performed according to the patent application agreement with KYOTO MEDICAL PLANNING Co., Ltd.
Figures





Similar articles
-
Ideal Duration of Pretreatment Using a Gelatin Hydrogel Nonwoven Fabric Prior to Subcutaneous Islet Transplantation.Cell Transplant. 2023 Jan-Dec;32:9636897231186063. doi: 10.1177/09636897231186063. Cell Transplant. 2023. PMID: 37466120 Free PMC article.
-
A gelatin hydrogel nonwoven fabric improves outcomes of subcutaneous islet transplantation.Sci Rep. 2023 Jul 24;13(1):11968. doi: 10.1038/s41598-023-39212-4. Sci Rep. 2023. PMID: 37488155 Free PMC article.
-
A Gelatin Hydrogel Nonwoven Fabric Enhances Subcutaneous Islet Engraftment in Rats.Cells. 2023 Dec 26;13(1):51. doi: 10.3390/cells13010051. Cells. 2023. PMID: 38201255 Free PMC article.
-
A novel method of pancreatic islet transplantation at the liver surface using a gelatin hydrogel nonwoven fabric.Cell Transplant. 2025 Jan-Dec;34:9636897251328419. doi: 10.1177/09636897251328419. Epub 2025 Apr 23. Cell Transplant. 2025. PMID: 40264358 Free PMC article.
-
Trophic effects of adipose derived stem cells on Langerhans islets viability--Review.Transplant Rev (Orlando). 2015 Jul;29(3):121-6. doi: 10.1016/j.trre.2015.04.006. Epub 2015 May 12. Transplant Rev (Orlando). 2015. PMID: 26002997 Review.
Cited by
-
A novel subcutaneous islet transplantation method using a bioabsorbable medical device to facilitate the creation of a highly vascularized transplantation site.Cell Transplant. 2025 Jan-Dec;34:9636897251342986. doi: 10.1177/09636897251342986. Epub 2025 Jun 2. Cell Transplant. 2025. PMID: 40454554 Free PMC article.
-
A novel approach for hepatocyte transplantation at the liver surface.Cell Transplant. 2025 Jan-Dec;34:9636897251329308. doi: 10.1177/09636897251329308. Epub 2025 Apr 10. Cell Transplant. 2025. PMID: 40208805 Free PMC article.
References
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–38. - PubMed
-
- Anazawa T, Saito T, Goto M, Kenmochi T, Uemoto S, Itoh T, Yasunami Y, Kenjo A, Kimura T, Ise K, Tsuchiya T, et al.. Long-term outcomes of clinical transplantation of pancreatic islets with uncontrolled donors after cardiac death: a multicenter experience in Japan. Transplant Proc. 2014;46(6):1980–84. - PubMed
-
- Goto M, Johansson U, Eich TM, Lundgrem T, Engkvist M, Felldin M, Foss A, Kallen R, Salmela K, Tibell A, Tufveson G, et al.. Key factors for human islet isolation and clinical transplantation. Transplant Proc. 2005;37(2):1315–16. - PubMed
-
- Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77(5):741–47. - PubMed
-
- Tokodai K, Goto M, Inagaki A, Nakanishi W, Okada N, Okada H, Satomi S. C5a-inhibitory peptide combined with gabexate mesilate prevents the instant blood-mediated inflammatory reaction in a rat model of islet transplantation. Transplant Proc. 2010;42(6):2102–103. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

