Female Alms1-deficient mice develop echocardiographic features of adult but not infantile Alström syndrome cardiomyopathy
- PMID: 38756069
- PMCID: PMC11225586
- DOI: 10.1242/dmm.050561
Female Alms1-deficient mice develop echocardiographic features of adult but not infantile Alström syndrome cardiomyopathy
Abstract
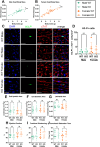
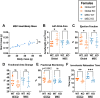
Alström syndrome (AS), a multisystem disorder caused by biallelic ALMS1 mutations, features major early morbidity and mortality due to cardiac complications. The latter are biphasic, including infantile dilated cardiomyopathy and distinct adult-onset cardiomyopathy, and poorly understood. We assessed cardiac function of Alms1 knockout (KO) mice by echocardiography. Cardiac function was unaltered in Alms1 global KO mice of both sexes at postnatal day 15 (P15) and 8 weeks. At 23 weeks, female - but not male - KO mice showed increased left atrial area and decreased isovolumic relaxation time, consistent with early restrictive cardiomyopathy, as well as reduced ejection fraction. No histological or transcriptional changes were seen in myocardium of 23-week-old female Alms1 global KO mice. Female mice with Pdgfra-Cre-driven Alms1 deletion in cardiac fibroblasts and in a small proportion of cardiomyocytes did not recapitulate the phenotype of global KO at 23 weeks. In conclusion, only female Alms1-deficient adult mice show echocardiographic evidence of cardiac dysfunction, consistent with the cardiomyopathy of AS. The explanation for sexual dimorphism remains unclear but might involve metabolic or endocrine differences between sexes.
Keywords: ALMS1; Alstrom syndrome; Alström syndrome; Cardiomyopathy; Ciliopathy; Heart; Primary cilia.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests R.K.S. has received consulting fees from Novartis, Astra Zeneca and Alnylam, research contribution in kind from Pfizer, and speaking fees from Novo Nordisk, Eli Lilly and Amryt.
Figures


References
-
- Arsov, T., Silva, D. G., O'Bryan, M. K., Sainsbury, A., Lee, N. J., Kennedy, C., Manji, S. S. M., Nelms, K., Liu, C., Vinuesa, C. G.et al. (2006b). Fat aussie - a new Alström syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol. Endocrinol. 20, 1610-1622. 10.1210/me.2005-0494 - DOI - PubMed
-
- Baig, S., Veeranna, V., Bolton, S., Edwards, N., Tomlinson, J. W., Manolopoulos, K., Moran, J., Steeds, R. P. and Geberhiwot, T. (2018). Treatment with PBI-4050 in patients with Alström syndrome: study protocol for a phase 2, single-Centre, single-arm, open-label trial. BMC Endocr. Disord. 18, 88. 10.1186/s12902-018-0315-6 - DOI - PMC - PubMed
-
- Baig, S., Dowd, R., Edwards, N. C., Hodson, J., Fabritz, L., Vijapurapu, R., Liu, B., Geberhiwot, T. and Steeds, R. P. (2020b). Prospective cardiovascular magnetic resonance imaging in adults with Alström syndrome: silent progression of diffuse interstitial fibrosis. Orphanet J. Rare Dis. 15, 139. 10.1186/s13023-020-01426-4 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

