PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma
- PMID: 38756145
- PMCID: PMC11095598
- DOI: 10.1159/000533650
PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma
Abstract
Introduction: Intratumoral administration of pexa-vec (pexastimogene devacirepvec), an oncolytic and immunotherapeutic vaccinia virus, given to patients with hepatocellular carcinoma (HCC), is associated with both local and distant tumor responses. We hypothesized subsequent treatment with sorafenib could demonstrate superior efficacy.
Methods: This random phase III open-label study evaluated the sequential treatment with pexa-vec followed by sorafenib compared to sorafenib in patients with advanced HCC and no prior systemic treatment. The primary endpoint is overall survival (OS). Key secondary endpoints included time to progression (TTP), progression-free survival, overall response rate (ORR), and disease control rate (DCR). Safety was assessed in all patients who received ≥1 dose of study treatment.
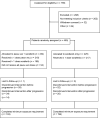
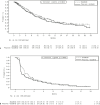
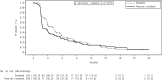
Results: The study was conducted at 142 sites in 16 countries. From December 30, 2015, to the interim analysis on August 2, 2019, 459 patients were randomly assigned (pexa-vec plus sorafenib: 234, sorafenib: 225). At the interim analysis, the median OS was 12.7 months (95% CI: 9.89, 14.95) in the pexa-vec plus sorafenib arm and 14.0 months (95% CI: 11.01, 18.00) in the sorafenib arm. This led to the early termination of the study. The median TTP was 2.0 months (95% CI: 1.77, 2.96) and 4.2 months (95% CI: 2.92, 4.63); ORR was 19.2% (45 patients) and 20.9% (47 patients); and DCR was 50.0% (117 patients) and 57.3% (129 patients) in the pexa-vec plus sorafenib and sorafenib arms, respectively. Serious adverse events were reported in 117 (53.7%) patients in the pexa-vec plus sorafenib and 77 (35.5%) patients in the sorafenib arm. Liver failure was the most frequently reported in both groups.
Conclusion: Sequential pexa-vec plus sorafenib treatment did not demonstrate increased clinical benefit in advanced HCC and fared worse compared to sorafenib alone. The advent of the added value of checkpoint inhibitors should direct any further development of oncolytic virus therapy strategies.
Keywords: Hepatocellular carcinoma; JX-594; Pexa-vec; Sorafenib.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Ghassan K. Abou-Alfa reports research support from Arcus, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed, Yiviva, and consulting support from Adicet, Alnylam, Astra Zeneca, Autem, Beigene, Berry Genomics, Boehringer Ingelheim, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Helsinn, Incyte, Ipsen, Merck, Nerviano, Newbridge, Novartis, QED, Redhill, Rafael, Servier, Silenseed, Sobi, Vector, Yiviva, plus patent PCT/US2014/031545 filed on March 24, 2014, and priority application Serial No.: 61/804,907; filed: March 25, 2013. Peter R. Galle reports research support from Bayer and Roche and consulting support from Bayer, Boston Scientific, AstraZeneca, Adaptimmune, BMS, Eisai, MSD, Sirtex, Lilly, Roche, Guerbet, and Ipsen. Joseph Erinjeri reports consulting support from AztraZeneca. Jeong Heo received grants/research support from Roche, Yuhan, and Gilead and participates on trials steering committee for Sillajen, Oncolys, Gilead, and AstraZeneca. Mitesh J. Borad report research support from Senhwa Pharmaceuticals, Adaptimmune, Agios Pharmaceuticals, Halozyme Pharmaceuticals, Celgene Pharmaceuticals, EMD Merck Serono, Toray, Dicerna, Taiho Pharmaceuticals, Sun Biopharma, Isis Pharmaceuticals, Redhill Pharmaceuticals, Boston Biomed, Basilea, Incyte Pharmaceuticals, Mirna Pharmaceuticals, Medimmune, Bioline, Sillajen, ARIAD Pharmaceuticals, PUMA Pharmaceuticals, Novartis Pharmaceuticals, QED Pharmaceuticals, Pieris Pharmaceuticals, and consulting support from ADC Therapeutics, Exelixis Pharmaceuticals, Inspyr Therapeutics, G1 Therapeutics, Immunovative Therapies, OncBioMune Pharmaceuticals, Western Oncolytics, Lynx Group, Genentech, Merck, Huya. James Burke is employed by CG Oncology and is consultant for Oncomyx, Kalivir, Western Oncolytics, Sonata, and Amped. Adina Pelusio is employed by Kalivir Immunotherapeutics and reports previous employment by SillaJen Biotherapeutics and Turnstone Biologics, Corp. Delphine Agathon reports previous employment by Transgene. Caroline Breitbach is an inventor on SillaJen patents. Edward Gane is a member of the Scientific Advisory Boards as an advisor and/or speaker for AbbVie, Abbott Diagnostics, Aligos, Arbutus, Arrowhead, Assembly, Dicerna, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Novartis, Roche, Surrozen, The Liver Company, Vaccitech, Virion Therapeutics, and Vir Bio and is on Speakers’ Bureau for AbbVie and Abbott Diagnostics. Yee Chao, Angelo Luca, Monika Lusky, and Shukui Qin report no conflicts of interest.
Figures

References
-
- World Health Organization International Agency for Research on Cancer (IARC) . GLOBOCAN 2020: Liver. [cited 2021 Apr 27].
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med Overseas Ed. 2008 Jul 24;359(4):378–90. - PubMed
-
- Kudo M, Finn RS, Qin S, Han K, Ikeda K, Piscaglia F, et al. . Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163–73. - PubMed
-
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. . IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_Suppl):267.
Grants and funding
LinkOut - more resources
Full Text Sources

