Safety and Efficacy of Atezolizumab/Bevacizumab in Patients with Hepatocellular Carcinoma and Impaired Liver Function: A Systematic Review and Meta-Analysis
- PMID: 38756146
- PMCID: PMC11095597
- DOI: 10.1159/000533991
Safety and Efficacy of Atezolizumab/Bevacizumab in Patients with Hepatocellular Carcinoma and Impaired Liver Function: A Systematic Review and Meta-Analysis
Abstract
Background: Safety and outcome of atezolizumab/bevacizumab in Child-Pugh B patients with hepatocellular carcinoma (HCC) have not been completely characterized.
Objectives: In this study, we aimed at addressing safety and efficacy of atezolizumab/bevacizumab in Child-Pugh B patients by reviewing the available data and analyzing them by meta-analysis.
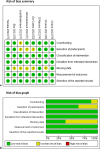
Methods: We compared the safety and efficacy of atezolizumab/becavizumab treatment in patients with unresectable HCC and various degrees of liver dysfunction. A total of 8 retrospective, non-randomized, cohort studies were included in this meta-analysis, for a total of 1,071 Child-Pugh A and 225 Child-Pugh B patients. The albumin-bilirubin (ALBI) grade was also used to assess liver function, when available.
Results: Grade ≥3 adverse events were observed in 11.8% of Child-Pugh class A and 26.8% class B patients (p = 0.0001), with an odds ratio (OR) of 0.43 (confidence interval [CI] 0.21-0.90; p = 0.02). Progression-free survival (PFS) at both 6 months (4.90 ± 2.08 vs. 4.75 ± 2.08 months; p = 0.0004) and 12 months (8.83 ± 2.32 vs. 7.26 ± 2.33 months; p = 0.002) was lower in Child-Pugh class B patients. A trend toward a higher objective response rate (ORR) was observed in Child-Pugh class A patients (219/856, 25.6%) as compared to Child-Pugh class B patients (25/138, 18.1%; p = 0.070), while the probability of obtaining an ORR was significantly greater in Child-Pugh A patients (OR 1.79, CI 1.12-2.86; p = 0.02). Median overall survival (OS) was 16.8 ± 2.0 and 6.8 ± 3.2 months in Child-Pugh A and B patients, respectively (mean difference 9.06 months, CI 7.01-11.1, p < 0.0001). Lastly, OS was longer in patients with ALBI grades 1-2 than in those with grade 3 (8.3 ± 11.4 vs. 3.3 ± 5.0 months, p = 0.0008).
Conclusions: Oncological efficacy of atezolizumab/bevacizumab is moderate in Child-Pugh class B patients, and the shorter PFS and OS associated with the greater likelihood of experiencing treatment-related adverse events observed in these patients suggest great caution and individualization of treatment, possibly with the support of the ALBI grade.
Keywords: Benefit; Cirrhosis; Survival; Systemic treatment.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Edoardo G. Giannini has participated in consulting and/or advisory boards for Roche, AstraZeneca, Eisai, MSD. Mario Strazzabosco is an advisor for ENGITIX.
Figures




References
-
- Garuti F, Neri A, Avanzato F, Gramenzi A, Rampoldi D, Rucci P, et al. . The changing scenario of hepatocellular carcinoma in Italy: an update. Liver Int. 2021;41(3):585–97. - PubMed
-
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. - PubMed
-
- Jeon D, Song G-W, Lee HC, Shim JH. Treatment patterns for hepatocellular carcinoma in patients with Child-Pugh class B and their impact on survival: a Korean nationwide registry study. Liver Int. 2022;42(12):2830–42. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

