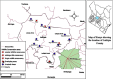
Molecular Detection of Anaplasma phagocytophilum in Small Mammals and Infesting Ticks in Laikipia County, Kenya
- PMID: 38756415
- PMCID: PMC11098608
- DOI: 10.1155/2024/5575162
Molecular Detection of Anaplasma phagocytophilum in Small Mammals and Infesting Ticks in Laikipia County, Kenya
Abstract
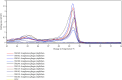
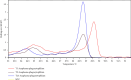
Anaplasmosis is a set of disease conditions of various mammals caused by bacteria species of the genus Anaplasma. These are sub-microscopic, Gram-negative, obligate intracellular pathogens that infect both vertebrate and invertebrate hosts. Significant species that infect domestic and wildlife animals include Anaplasma marginale, Anaplasma ovis, Anaplasma mesaeterum, Anaplasma platys, and Anaplasma phagocytophilum. Although A. phagocytophilum has a widespread distribution, there are only a few epidemiological reports from sub-Saharan Africa. This study focused on molecular detection and characterization of A. phagocytophilum in small mammals and their infesting ticks in Laikipia County, Kenya. A total of 385 blood and 84 tick archival samples from small mammals (155 females and 230 males) were analyzed. The blood samples were subjected to a nested PCR-HRM melt analysis using species-specific primers to amplify the 16S ribosomal RNA genes. The ticks were also subjected to nested PCR-HRM involving 16S rRNA gene primers. Anaplasma phagocytophilum DNA was detected in 19 out of 385 samples using species-specific 16S rRNA gene primers giving a prevalence of 4.9% for A. phagocytophilum. Analysis of the tick's samples using 16S rRNA gene species-specific primers also detected A. phagocytophilum in 3 samples from Haemaphysalis leachi ticks (3/84) equivalent to prevalence of 3.6%. Sequencing of 16S rRNA PCR products confirmed A. phagocytophilum in small mammals and ticks' samples. Phylogenetic analysis of the haplotype from this study demonstrated a close ancestral link with strains from Canis lupus familiaris, Alces alces, Apodemus agrarius, and ticks (Haemaphysalis longicornis) reported in Europe, China, and Africa. Comparison was also made with a known pathogenic A. phagocytophilum variant HA and a nonpathogenic variant 1 that were clustered into a distinctive clade different form haplotypes detected in this study. All the haplotype sequences for A. phagocytophilum from this study were submitted and registered in GenBank under the accession numbers OQ308965-OQ308976. Our study shows that small mammals and their associated ticks harbor A. phagocytophilum. The vector competence for H. leachi in A. phagocytophilum transmission should further be investigated.
Copyright © 2024 Erick Titus Mosha et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Dzięgiel B., Adaszek Ł., Winiarczyk S. Wild animals as reservoirs of Anaplasma phagocytophilum for humans. Przeglad Epidemiologiczny . 2016;70(3):428–435. - PubMed
-
- Dumler J. S., Barbet A. F., Bekker C. P. J., et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. International Journal of Systematic and Evolutionary Microbiology . 2001;51(6):2145–2165. doi: 10.1099/00207713-51-6-2145. - DOI - PubMed
LinkOut - more resources
Full Text Sources

