Imidazole functionalized photo-crosslinked aliphatic polycarbonate drug-eluting coatings on zinc alloys for osteogenesis, angiogenesis, and bacteriostasis in bone regeneration
- PMID: 38756420
- PMCID: PMC11096721
- DOI: 10.1016/j.bioactmat.2024.03.037
Imidazole functionalized photo-crosslinked aliphatic polycarbonate drug-eluting coatings on zinc alloys for osteogenesis, angiogenesis, and bacteriostasis in bone regeneration
Abstract
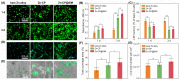
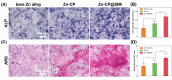
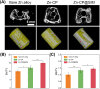
Zinc (Zn) alloys have demonstrated significant potential in healing critical-sized bone defects. However, the clinical application of Zn alloys implants is still hindered by challenges including excessive release of zinc ions (Zn2+), particularly in the early stage of implantation, and absence of bio-functions related to complex bone repair processes. Herein, a biodegradable aliphatic polycarbonate drug-eluting coating was fabricated on zinc-lithium (Zn-Li) alloys to inhibit Zn2+ release and enhance the osteogenesis, angiogenesis, and bacteriostasis of Zn alloys. Specifically, the photo-curable aliphatic polycarbonates were co-assembled with simvastatin and deposited onto Zn alloys to produce a drug-loaded coating, which was crosslinked by subsequent UV light irradiation. During the 60 days long-term immersion test, the coating showed distinguished stable drug release and Zn2+ release inhibition properties. Benefiting from the regulated release of Zn2+ and simvastatin, the coating facilitated the adhesion, proliferation, and differentiation of MC3T3-E1 cells, as well as the migration and tube formation of EA.hy926 cells. Astonishingly, the coating also showed remarkable antibacterial properties against both S. aureus and E. coli. The in vivo rabbit critical-size femur bone defects model demonstrated that the drug-eluting coating could efficiently promote new bone formation and the expression of platelet endothelial cell adhesion molecule-1 (CD31) and osteocalcin (OCN). The enhancement of osteogenesis, angiogenesis, and bacteriostasis is achieved by precisely controlling of the released Zn2+ at an appropriate level, as well as the stable release profile of simvastatin. This tailored aliphatic polycarbonate drug-eluting coating provides significant potential for clinical applications of Zn alloys implants.
Keywords: Aliphatic polycarbonate; Angiogenesis; Drug-eluting coating; Osteogenesis; Zn alloy implant.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Facile fabrication of biodegradable endothelium-mimicking coatings on bioabsorbable zinc-alloy stents by one-step electrophoretic deposition.J Mater Chem B. 2022 Apr 20;10(16):3083-3096. doi: 10.1039/d2tb00119e. J Mater Chem B. 2022. PMID: 35343560
-
Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31.Acta Biomater. 2019 Oct 15;98:196-214. doi: 10.1016/j.actbio.2019.05.069. Epub 2019 May 31. Acta Biomater. 2019. PMID: 31154057
-
Zinc Ion-crosslinked polycarbonate/heparin composite coatings for biodegradable Zn-alloy stent applications.Colloids Surf B Biointerfaces. 2022 Oct;218:112725. doi: 10.1016/j.colsurfb.2022.112725. Epub 2022 Jul 25. Colloids Surf B Biointerfaces. 2022. PMID: 35914466
-
Biofunctional magnesium coating of implant materials by physical vapour deposition.Biomater Transl. 2021 Sep 28;2(3):248-256. doi: 10.12336/biomatertransl.2021.03.007. eCollection 2021. Biomater Transl. 2021. PMID: 35836651 Free PMC article. Review.
-
Progress in bioactive surface coatings on biodegradable Mg alloys: A critical review towards clinical translation.Bioact Mater. 2022 May 15;19:717-757. doi: 10.1016/j.bioactmat.2022.05.009. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35633903 Free PMC article. Review.
Cited by
-
Photothermal Coating on Zinc Alloy for Controlled Biodegradation and Improved Osseointegration.Adv Sci (Weinh). 2025 Mar;12(9):e2409051. doi: 10.1002/advs.202409051. Epub 2025 Jan 14. Adv Sci (Weinh). 2025. PMID: 39807526 Free PMC article.
-
Photothermally controlled ICG@ZIF-8/PLGA coating to modify the degradation behavior and biocompatibility of Zn-Li alloy for bone implants.Regen Biomater. 2025 Jan 6;12:rbaf001. doi: 10.1093/rb/rbaf001. eCollection 2025. Regen Biomater. 2025. PMID: 40040751 Free PMC article.
References
-
- Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5(8):584–603.
-
- Stevens M.M. Biomaterials for bone tissue engineering. Mater. Today. 2008;11(5):18–25.
LinkOut - more resources
Full Text Sources

