Rational design and investigation of nonlinear optical response properties of pyrrolopyrrole aza-BODIPY-based novel push-pull chromophores
- PMID: 38756482
- PMCID: PMC11097754
- DOI: 10.1039/d4ra02861a
Rational design and investigation of nonlinear optical response properties of pyrrolopyrrole aza-BODIPY-based novel push-pull chromophores
Abstract
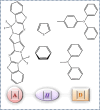
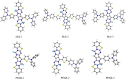
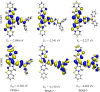
Intramolecular charge transfer (ICT)-based chromophores are highly sought after for designing near-infrared (NIR) absorbing and emitting dyes as well as for designing materials for nonlinear optical (NLO) applications. The properties of these 'push-pull' molecules can easily be modified by varying the electronic donor (D) and acceptor (A) groups as well as the π-conjugation linker. This study presents a methodical approach and employs quantum chemical analysis to explore the relationship between the structural features, electro-optical properties, and the NLO characteristics of molecules with D-π-A framework. The one- and two-photon absorption (2PA), linear polarizability (α), and first hyperpolarizability (β) of some novel chromophores, consisting of a dimeric aza-Boron Dipyrromethene (aza-BODIPY) analogue, called, pyrrolopyrrole aza-BODIPY (PPAB), serving as the acceptor, have been investigated. The electronic donors used in this study are triphenylamine (TPA) and diphenylamine (DPA), and they are conjugated to the acceptor via thienyl or phenylene π-linkers. Additionally, the Hyper-Rayleigh Scattering (βHRS), which enables direct estimation of the second-order NLO properties, is calculated for the studied chromophores with 1064 nm excitation in acetonitrile. The β value shows a significant increase with increasing solvent polarity, indicating that the ICT plays a crucial role in shaping the NLO response of the studied molecules. The enhancement of the 2PA cross-section of the investigated molecules can also be achieved by modulating the combinations of donors and linkers. The results of our study indicate that the novel D-π-A molecules designed in this work demonstrate considerably higher hyperpolarizability values than the standard p-nitroaniline, making them promising candidates for future NLO applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Structural modulation of π-conjugated linkers in D-π-A dyes based on triphenylamine dicyanovinylene framework to explore the NLO properties.R Soc Open Sci. 2021 Aug 4;8(8):210570. doi: 10.1098/rsos.210570. eCollection 2021 Aug. R Soc Open Sci. 2021. PMID: 34386260 Free PMC article.
-
The role of π-linkers and electron acceptors in tuning the nonlinear optical properties of BODIPY-based zwitterionic molecules.RSC Adv. 2020 Nov 5;10(66):40300-40309. doi: 10.1039/d0ra02193h. eCollection 2020 Nov 2. RSC Adv. 2020. PMID: 35520880 Free PMC article.
-
Tuning of hyperpolarizability, and one- and two-photon absorption of donor-acceptor and donor-acceptor-acceptor-type intramolecular charge transfer-based sensors.Phys Chem Chem Phys. 2019 Aug 21;21(31):17343-17355. doi: 10.1039/c9cp03772a. Epub 2019 Jul 29. Phys Chem Chem Phys. 2019. PMID: 31355378
-
Second-order nonlinear polarizability of "Push-Pull" chromophores. A decade of progress in donor-π-acceptor materials.Chem Rec. 2022 Jun;22(6):e202200024. doi: 10.1002/tcr.202200024. Epub 2022 Mar 29. Chem Rec. 2022. PMID: 35352466 Review.
-
Roadmap for Designing Donor-π-Acceptor Fluorophores in UV-Vis and NIR Regions: Synthesis, Optical Properties and Applications.Biomolecules. 2025 Jan 14;15(1):119. doi: 10.3390/biom15010119. Biomolecules. 2025. PMID: 39858513 Free PMC article. Review.
References
-
- Yu J. Luo M. Lv Z. Huang S. Hsu H. H. Kuo C. C. Han S. T. Zhou Y. Nanoscale. 2020;12:23391–23423. - PubMed
-
- Dalton L. R. Sullivan P. A. Bale D. H. Chem. Rev. 2010;110:25–55. - PubMed
-
- Yang L. El-Tamer A. Hinze U. Li J. Hu Y. Huang W. Chu J. Chichkov B. N. Appl. Phys. Lett. 2014;105:041110.
-
- Khalid M. Lodhi H. M. Khan M. U. Imran M. RSC Adv. 2021;11:14237–14250.
-
- Feng Q. Li Y. Shi G. Wang L. Zhang W. Li K. Hou H. Song Y. J. Mater. Chem. C. 2016;4:8552–8558.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

