KRAS G12C inhibitor combination therapies: current evidence and challenge
- PMID: 38756650
- PMCID: PMC11097198
- DOI: 10.3389/fonc.2024.1380584
KRAS G12C inhibitor combination therapies: current evidence and challenge
Abstract
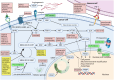
Although KRAS G12C inhibitors have proven that KRAS is a "druggable" target of cancer, KRAS G12C inhibitor monotherapies have demonstrated limited clinical efficacy due to primary and acquired resistance mechanisms. Multiple combinations of KRAS G12C inhibitors with other targeted therapies, such as RTK, SHP2, and MEK inhibitors, have been investigated in clinical trials to overcome the resistance. They have demonstrated promising efficacy especially by combining KRAS G12C and EGFR inhibitors for KRAS G12C-mutated colorectal cancer. Many clinical trials of combinations of KRAS G12C inhibitors with other targeted therapies, such as SOS1, ERK, CDK4/6, and wild-type RAS, are ongoing. Furthermore, preclinical data have suggested additional promising KRAS G12C combinations with YAP/TAZ-TEAD inhibitors, FAK inhibitors, and farnesyltransferase inhibitors. The combinations of KRAS G12C inhibitors with immunotherapies and chemotherapies have also been investigated, and the preliminary results were reported. More recently, KRAS-targeted therapies not limited to KRAS G12C are being developed, potentially broadening the treatment landscape of KRAS-mutated cancers. Rationally combining KRAS inhibitors with other therapeutics is likely to play a significant role in future treatment for KRAS-mutated solid tumors.
Keywords: KRAS; KRAS G12C inhibitors; MAPK; RTK inhibitors; combination (combined) therapy; immunotherapy; mTOR.
Copyright © 2024 Miyashita, Kato and Hong.
Conflict of interest statement
SK serves as a consultant for Medpace, Foundation Medicine, NeoGenomics and CureMatch. He receives speaker’s fee from Roche/Genentech and Bayer, and advisory board for Pfizer. He has research funding from ACT Genomics, Sysmex, Konica Minolta, OmniSeq, Personalis and Function Oncology. DH discloses the following that could be potential conflict of interest: Research (Inst)/Grant Funding (Inst) AbbVie, Adaptimmune, Adlai-Nortye, Amgen, Astelles, Astra-Zeneca, Bayer, Biomea, Bristol-Myers Squibb, Daiichi-Sankyo, Deciphera, Eisai, Eli Lilly, Endeavor, Erasca, F. Hoffmann-LaRoche, Fate Therapeutics, Genentech, Genmab, Immunogenesis, Infinity, Kyowa Kirin, Merck, Mirati, Navier, NCI-CTEP, Novartis, Numab, Pfizer, Pyramid Bio, Revolution Medicine, SeaGen, STCube, Takeda, TCR2, Turning Point Therapeutics, VM Oncology. Travel, Accommodations, Expenses: AACR, ASCO, CLCC, Bayer, Genmab, SITC, Telperian. Consulting, Speaker, or Advisory Role: 28Bio, Abbvie, Acuta, Adaptimmune, Alkermes, Alpha Insights, Amgen, Affini-T, Astellas, Aumbiosciences, Axiom, Baxter, Bayer, Boxer Capital, BridgeBio, CARSgen, CLCC, COG, COR2ed, Cowen, Ecor1, EDDC, Erasca, Exelixis, Fate Therapeutics, F.Hoffmann-La Roche, Genentech, Gennao Bio, Gilead, GLG, Group H, Guidepoint, HCW Precision Oncology, Immunogenesis, Incyte Inc, Inhibrix Inc, InduPro, Janssen, Jounce Therapeutics Inc,Lan- Bio, Liberium, MedaCorp, Medscape, Novartis, Numab, Oncologia Brasil, ORI Capital, Pfizer, Pharma Intelligence, POET Congress, Prime Oncology, Projects in Knowledge, Quanta, RAIN, Ridgeline, Revolution Medicine SeaGen, Stanford, STCube, Takeda, Tavistock, Trieza Therapeutics,T-Knife, Turning Point Therapeutics, WebMD, YingLing Pharma, Ziopharm. Other ownership interests: Molecular Match (Advisor), OncoResponse (Founder, Advisor), Telperian (Founder, Advisor). The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

