Long-term humoral and cellular immunity against vaccine strains and Omicron subvariants (BQ.1.1, BN.1, XBB.1, and EG.5) after bivalent COVID-19 vaccination
- PMID: 38756783
- PMCID: PMC11096540
- DOI: 10.3389/fimmu.2024.1385135
Long-term humoral and cellular immunity against vaccine strains and Omicron subvariants (BQ.1.1, BN.1, XBB.1, and EG.5) after bivalent COVID-19 vaccination
Abstract
Background: The assessment of long-term humoral and cellular immunity post-vaccination is crucial for establishing an optimal vaccination strategy.
Methods: This prospective cohort study evaluated adults (≥18 years) who received a BA.4/5 bivalent vaccine. We measured the anti-receptor binding domain immunoglobulin G antibody and neutralizing antibodies (NAb) against wild-type and Omicron subvariants (BA.5, BQ.1.1, BN.1, XBB.1 and EG.5) up to 9 months post-vaccination. T-cell immune responses were measured before and 4 weeks after vaccination.
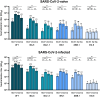
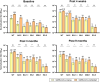
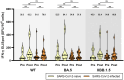
Results: A total of 108 (28 SARS-CoV-2-naïve and 80 previously infected) participants were enrolled. Anti-receptor binding domain immunoglobulin G (U/mL) levels were higher at 9 months post-vaccination than baseline in SAR-CoV-2-naïve individuals (8,339 vs. 1,834, p<0.001). NAb titers against BQ.1.1, BN.1, and XBB.1 were significantly higher at 9 months post-vaccination than baseline in both groups, whereas NAb against EG.5 was negligible at all time points. The T-cell immune response (median spot forming unit/106 cells) was highly cross-reactive at both baseline (wild-type/BA.5/XBB.1.5, 38.3/52.5/45.0 in SARS-CoV-2-naïve individuals; 51.6/54.9/54.9 in SARS-CoV-2-infected individuals) and 4 weeks post-vaccination, with insignificant boosting post-vaccination.
Conclusion: Remarkable cross-reactive neutralization was observed against BQ.1.1, BN.1, and XBB.1 up to 9 months after BA.4/5 bivalent vaccination, but not against EG.5. The T-cell immune response was highly cross-reactive.
Keywords: EG.5; SARS-CoV-2; cellular immunity; cross-reactivity; durability; humoral immunity; vaccines.
Copyright © 2024 Hyun, Nham, Seong, Yoon, Noh, Cheong, Kim, Yoon, Park, Gwak, Lee, Kim and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization . WHO COVID-19 dashboard(2024). Available online at: https://data.who.int/dashboards/covid19/cases?n=c (Accessed 12 February 2024).
-
- Hodcroft EB. CoVariants: SARS-CoV-2 mutations and variants of interest(2024). Available online at: https://covariants.org/per-country?region=World (Accessed 25 January 2024).
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

