Mild chemistry synthesis of ultrathin Bi2O2S nanosheets exhibiting 2D-ferroelectricity at room temperature
- PMID: 38756816
- PMCID: PMC11095514
- DOI: 10.1039/d4sc00067f
Mild chemistry synthesis of ultrathin Bi2O2S nanosheets exhibiting 2D-ferroelectricity at room temperature
Abstract
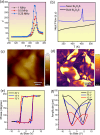
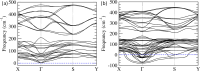
Modern technology demands miniaturization of electronic components to build small, light, and portable devices. Hence, discovery and synthesis of new non-toxic, low cost, ultra-thin ferroelectric materials having potential applications in various electronic and optoelectronic devices are of paramount importance. However, achieving room-temperature ferroelectricity in two dimensional (2D) ultra-thin systems remains a major challenge as conventional three-dimensional ferroelectric materials lose their ferroelectricity when the thickness is brought down below a critical value owing to the depolarization field. Herein, we report room-temperature ferroelectricity in ultra-thin single-crystalline 2D nanosheets of Bi2O2S synthesized by a simple, rapid, and scalable solution-based soft chemistry method. The ferroelectric ground state of Bi2O2S nanosheets is confirmed by temperature-dependent dielectric measurements as well as piezoelectric force microscopy and spectroscopy. High resolution transmission electron microscopy analysis and density functional theory-based calculations suggest that the ferroelectricity in Bi2O2S nanosheets arises due to the local distortion of Bi2O2 layers, which destroys the local inversion symmetry of Bi2O2S.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Hong H. Wu C. Zhao Z. Zuo Y. Wang J. Liu C. Zhang J. Wang F. Feng J. Shen H. Yin J. Wu Y. Zhao Y. Liu K. Gao P. Meng S. Wu S. Sun Z. Liu K. Xiong J. Nat. Photonics. 2021;15:510–515. doi: 10.1038/s41566-021-00801-2. - DOI
-
- Sun Z. Martinez A. Wang F. Nat. Photonics. 2016;10:227–238. doi: 10.1038/nphoton.2016.15. - DOI
LinkOut - more resources
Full Text Sources

