Incidence and Mitigation of Corneal Pseudomicrocysts Induced by Antibody-Drug Conjugates (ADCs)
- PMID: 38756824
- PMCID: PMC11095972
- DOI: 10.1007/s40135-024-00322-5
Incidence and Mitigation of Corneal Pseudomicrocysts Induced by Antibody-Drug Conjugates (ADCs)
Abstract
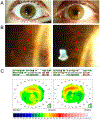
Purpose of review: This study is to highlight the incidence of corneal pseudomicrocysts in FDA-approved antibody-drug conjugates (ADCs), and success of preventive therapies for pseudomicrocysts and related ocular surface adverse events (AEs).
Recent findings: ADCs are an emerging class of selective cancer therapies that consist of a potent cytotoxin connected to a monoclonal antibody (mAb) that targets antigens expressed on malignant cells. Currently, there are 11 FDA-approved ADCs with over 164 in clinical trials. Various AEs have been attributed to ADCs, including ocular surface AEs (keratitis/keratopathy, dry eye, conjunctivitis, blurred vision, corneal pseudomicrocysts). While the severity and prevalence of ADC-induced ocular surface AEs are well reported, the reporting of corneal pseudomicrocysts is limited, complicating the development of therapies to prevent or treat ADC-related ocular surface toxicity.
Summary: Three of 11 FDA-approved ADCs have been implicated with corneal pseudomicrocysts, with incidence ranging from 41 to 100% of patients. Of the six ADCs that reported ocular surface AEs, only three had ocular substudies to investigate the benefit of preventive therapies including topical steroids, vasoconstrictors, and preservative-free lubricants. Current preventive therapies demonstrate limited efficacy at mitigating pseudomicrocysts and other ocular surface AEs.
Keywords: Antibody–drug conjugates; Conjunctiva; Cornea; Corneal pseudomicrocysts; Microcyst-like epithelial changes; Ocular surface adverse events; Ocular surface epithelium.
Conflict of interest statement
Conflict of Interest N.D.P. serves on the US Medical Keratopathy Advisory Board for Sanofi.
Figures
References
-
- Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023;22(8):641–61. - PubMed
-
Reviews the role of each ADC component in ocular surface toxicity.
-
- Jaffry M, Choudhry H, Aftab OM, Dastjerdi MH. Antibody-drug conjugates and ocular toxicity. J Ocul Pharmacol Ther. 2023;39(10):675–91. - PubMed
-
Highlights the potential mechanisms of ADC ocular surface toxicity and preventive therapies for FDA-approved ADCs as well as those in development.
-
- Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, Shinjo K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14(8):1436–43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
