Zirconium Oxynitride Thin Films for Photoelectrochemical Water Splitting
- PMID: 38756865
- PMCID: PMC11094725
- DOI: 10.1021/acsaem.4c00303
Zirconium Oxynitride Thin Films for Photoelectrochemical Water Splitting
Abstract
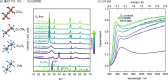
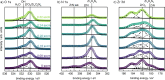
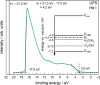
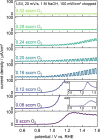
Transition metal oxynitrides are a promising class of functional materials for photoelectrochemical (PEC) applications. Although these compounds are most commonly synthesized via ammonolysis of oxide precursors, such synthetic routes often lead to poorly controlled oxygen-to-nitrogen anion ratios, and the harsh nitridation conditions are incompatible with many substrates, including transparent conductive oxides. Here, we report direct reactive sputter deposition of a family of zirconium oxynitride thin films and the comprehensive characterization of their tunable structural, optical, and functional PEC properties. Systematic increases of the oxygen content in the reactive sputter gas mixture enable access to different crystalline structures within the zirconium oxynitride family. Increasing oxygen contents lead to a transition from metallic to semiconducting to insulating phases. In particular, crystalline Zr2ON2-like films have band gaps in the UV-visible range and are n-type semiconductors. These properties, together with a valence band maximum position located favorably relative to the water oxidation potential, make them viable photoanode candidates. Using chopped linear sweep voltammetry, we indeed confirm that our Zr2ON2 films are PEC-active for the oxygen evolution reaction in alkaline electrolytes. We further show that high-vacuum annealing boosts their PEC performance characteristics. Although the observed photocurrents are low compared to state-of-the-art photoanodes, these dense and planar thin films can offer a valuable platform for studying oxynitride photoelectrodes, as well as for future nanostructuring, band gap engineering, and defect engineering efforts.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Brown J. J.; Ke Z.; Ma T.; Page A. J. Defect Engineering for Photocatalysis: From Ternary to Perovskite Oxynitrides. ChemNanoMat 2020, 6 (5), 708–719. 10.1002/cnma.201900703. - DOI
-
- Ahmed M.; Xinxin G. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3 (5), 578–590. 10.1039/C5QI00202H. - DOI
-
- Castelli I. E.; García-Lastra J. M.; Hüser F.; Thygesen K. S.; Jacobsen K. W. Stability and bandgaps of layered perovskites for one- and two-photon water splitting. New J. Phys. 2013, 15 (10), 105026.10.1088/1367-2630/15/10/105026. - DOI
-
- Sun W.; Bartel C. J.; Arca E.; Bauers S. R.; Matthews B.; Orvañanos B.; Chen B. R.; Toney M. F.; Schelhas L. T.; Tumas W.; Tate J.; Zakutayev A.; Lany S.; Holder A. M.; Ceder G. A map of the inorganic ternary metal nitrides. Nat. Mater. 2019, 18 (7), 732–739. 10.1038/s41563-019-0396-2. - DOI - PubMed
-
- Fuertes A. Synthetic approaches in oxynitride chemistry. Prog. Solid State Chem. 2018, 51, 63–70. 10.1016/j.progsolidstchem.2017.11.001. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
