Functional identification of DNA demethylase gene CaROS1 in pepper (Capsicum annuum L.) involved in salt stress
- PMID: 38756961
- PMCID: PMC11097670
- DOI: 10.3389/fpls.2024.1396902
Functional identification of DNA demethylase gene CaROS1 in pepper (Capsicum annuum L.) involved in salt stress
Abstract
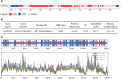
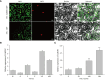
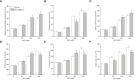
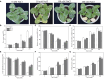
Pepper, which is a widely cultivated important vegetable, is sensitive to salt stress, and the continuous intensification of soil salinization has affected pepper production worldwide. However, genes confer to salt tolerance are rarely been cloned in pepper. Since the REPRESSOR OF SILENCING 1 (ROS1) is a DNA demethylase that plays a crucial regulatory role in plants in response to various abiotic stresses, including salt stress. We cloned a ROS1 gene in pepper, named CaROS1 (LOC107843637). Bioinformatic analysis showed that the CaROS1 protein contains the HhH-GPD glycosylase and RRM_DME domains. qRT-PCR analyses showed that the CaROS1 was highly expressed in young and mature fruits of pepper and rapidly induced by salt stress. Functional characterization of the CaROS1 was performed by gene silencing in pepper and overexpressing in tobacco, revealed that the CaROS1 positively regulates salt tolerance ability. More detailly, CaROS1-silenced pepper were more sensitive to salt stress, and their ROS levels, relative conductivity, and malondialdehyde content were significantly higher in leaves than those of the control plants. Besides, CaROS1-overexpressing tobacco plants were more tolerant to salt stress, with a higher relative water content, total chlorophyll content, and antioxidant enzyme activity in leaves compared to those of WT plants during salt stress. These results revealed the CaROS1 dose play a role in salt stress response, providing the theoretical basis for salt tolerance genetic engineering breeding in pepper.
Keywords: CaROS1; overexpression; pepper; salt stress; virus-induced gene silencing.
Copyright © 2024 Ou, Hua, Dong, Guo, Wu, Deng and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
CaDHN3, a Pepper (Capsicum annuum L.) Dehydrin Gene Enhances the Tolerance against Salt and Drought Stresses by Reducing ROS Accumulation.Int J Mol Sci. 2021 Mar 22;22(6):3205. doi: 10.3390/ijms22063205. Int J Mol Sci. 2021. PMID: 33809823 Free PMC article.
-
Transcription Factor CaSBP12 Negatively Regulates Salt Stress Tolerance in Pepper (Capsicum annuum L.).Int J Mol Sci. 2020 Jan 10;21(2):444. doi: 10.3390/ijms21020444. Int J Mol Sci. 2020. PMID: 31936712 Free PMC article.
-
CaFtsH06, A Novel Filamentous Thermosensitive Protease Gene, Is Involved in Heat, Salt, and Drought Stress Tolerance of Pepper (Capsicum annuum L.).Int J Mol Sci. 2021 Jun 28;22(13):6953. doi: 10.3390/ijms22136953. Int J Mol Sci. 2021. PMID: 34203346 Free PMC article.
-
Knockdown of CaHSP60-6 confers enhanced sensitivity to heat stress in pepper (Capsicum annuum L.).Planta. 2019 Dec;250(6):2127-2145. doi: 10.1007/s00425-019-03290-4. Epub 2019 Oct 12. Planta. 2019. PMID: 31606756
-
The SnRK2 family in pepper (Capsicum annuum L.): genome-wide identification and expression analyses during fruit development and under abiotic stress.Genes Genomics. 2020 Oct;42(10):1117-1130. doi: 10.1007/s13258-020-00968-y. Epub 2020 Jul 31. Genes Genomics. 2020. PMID: 32737808 Review.
References
-
- Ashraf M., Foolad M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. doi: 10.1016/j.envexpbot.2005.12.006 - DOI
-
- Ashraf M., Munns R. (2022). Evolution of approaches to increase the salt tolerance of crops. Crit. Rev. Plant Sci. 41, 128–160. doi: 10.1080/07352689.2022.2065136 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

