Ecophysiological responses of Phragmites australis populations to a tidal flat gradient in the Yangtze River Estuary, China
- PMID: 38756962
- PMCID: PMC11097105
- DOI: 10.3389/fpls.2024.1326345
Ecophysiological responses of Phragmites australis populations to a tidal flat gradient in the Yangtze River Estuary, China
Abstract
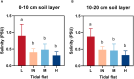
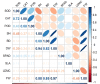
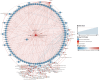
Phragmites australis is a prevalent species in the Chongming Dongtan wetland and is capable of thriving in various tidal flat environments, including high salinity habitats. P. australis population displays inconsistent ecological performances, highlighting the need to uncover their survival strategies and mechanisms in tidal flats with diverse soil salinities. Upon comparing functional traits of P. australis at multiple tidal flats (low, middle, and high) and their responses to soil physicochemical properties, this study aimed to clarify the salt-tolerant strategy of P. australis and the corresponding mechanisms. These results showed that leaf characteristics, such as specific leaf area and leaf dry matter content, demonstrated more robust stability to soil salinity than shoot height and dry weight. Furthermore, as salt stress intensified, the activities of superoxide dismutase (SOD), catalase (CAT) and peroxisome (POD) in P. australis leaves at low tidal flat exhibited an increased upward trend compared to those at other tidal flats. The molecular mechanism of salt tolerance in Phragmites australis across various habitats was investigated using transcriptome sequencing. Weighted correlation network analysis (WGCNA) combined with differentially expressed genes (DEGs) screened out 3 modules closely related to high salt tolerance and identified 105 core genes crucial for high salt tolerance. Further research was carried out on the few degraded populations at low tidal flat, and 25 core genes were identified by combining WGCNA and DEGs. A decrease in the activity of ferroptosis marker gonyautoxin-4 and an increase in the content of Fe3+ in the degenerated group were observed, indicating that ferroptosis might participate in degradation. Furthermore, correlation analysis indicated a possible regulatory network between salt tolerance and ferroptosis. In short, this study provided new insights into the salt tolerance mechanism of P. australis population along tidal flats.
Keywords: Phragmites australis; ferroptosis; salt tolerance; transcriptome; wetland ecosystem.
Copyright © 2024 Jia, Zhao, Jia, Zhang, Li, Liu and Huang.
Conflict of interest statement
Authors XCZ, XZ, and YL were employed by the company GeneMind Biosciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
[Effects of water table manipulation on leaf photosynthesis, morphology and growth of Phragmites australis and Imperata cylindrica in the reclaimed tidal wetland at Dongtan of Chongming Island, China].Ying Yong Sheng Tai Xue Bao. 2014 Feb;25(2):408-18. Ying Yong Sheng Tai Xue Bao. 2014. PMID: 24830240 Chinese.
-
Differentially expressed genes related to oxidoreductase activity and glutathione metabolism underlying the adaptation of Phragmites australis from the salt marsh in the Yellow River Delta, China.PeerJ. 2020 Oct 2;8:e10024. doi: 10.7717/peerj.10024. eCollection 2020. PeerJ. 2020. PMID: 33072439 Free PMC article.
-
[Responses of leaf functional traits of clonal plant Phragmites australis to heterogeneous environments].Ying Yong Sheng Tai Xue Bao. 2022 Aug;33(8):2171-2177. doi: 10.13287/j.1001-9332.202208.010. Ying Yong Sheng Tai Xue Bao. 2022. PMID: 36043824 Chinese.
-
[Effects of Water-salt Environment on Freshwater Wetland Soil C, N, and P Ecological Stoichiometric Characteristics in the Yellow River Estuary Wetland].Huan Jing Ke Xue. 2023 Aug 8;44(8):4698-4705. doi: 10.13227/j.hjkx.202209205. Huan Jing Ke Xue. 2023. PMID: 37694662 Chinese.
-
Cosmopolitan Species As Models for Ecophysiological Responses to Global Change: The Common Reed Phragmites australis.Front Plant Sci. 2017 Nov 16;8:1833. doi: 10.3389/fpls.2017.01833. eCollection 2017. Front Plant Sci. 2017. PMID: 29250081 Free PMC article. Review.
Cited by
-
How drought and ploidy level shape gene expression and DNA methylation in Phragmites australis.Plant Cell Rep. 2025 Aug 12;44(9):197. doi: 10.1007/s00299-025-03585-9. Plant Cell Rep. 2025. PMID: 40794332 Free PMC article.
References
-
- Ali A., Yun D.-J. (2017). Salt stress tolerance; what do we learn from halophytes? J. Plant Biol. 60, 431–439. doi: 10.1007/s12374-017-0133-9 - DOI
-
- An J. X., Wang Q., Yang J., Liu J. Q. (2012). Phylogeographic analyses of Phragmites australis in China: Native distribution and habitat preference of the haplotype that invaded North America. J. System. Evol. 50, 334–340. doi: 10.1111/j.1759-6831.2012.00192.x - DOI
-
- Angelini C., Altieri A. H., Silliman B. R., Bertness M. D. (2011). Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. BIOSCIENCE 61, 782–789. doi: 10.1525/bio.2011.61.10.8 - DOI
-
- Aslam R., Bostan N., Nabgha-e-Amen, Maria M., Safdar W. (2011). A critical review on halophytes: Salt tolerant plants. J. Med. Plants Res. 5, 7180–7118. doi: 10.5897/JMPRx11.009 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

