Host-microbiota interaction in intestinal stem cell homeostasis
- PMID: 38757687
- PMCID: PMC11110705
- DOI: 10.1080/19490976.2024.2353399
Host-microbiota interaction in intestinal stem cell homeostasis
Abstract
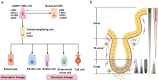
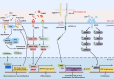
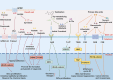
Intestinal stem cells (ISCs) play a pivotal role in gut physiology by governing intestinal epithelium renewal through the precise regulation of proliferation and differentiation. The gut microbiota interacts closely with the epithelium through myriad of actions, including immune and metabolic interactions, which translate into tight connections between microbial activity and ISC function. Given the diverse functions of the gut microbiota in affecting the metabolism of macronutrients and micronutrients, dietary nutrients exert pronounced effects on host-microbiota interactions and, consequently, the ISC fate. Therefore, understanding the intricate host-microbiota interaction in regulating ISC homeostasis is imperative for improving gut health. Here, we review recent advances in understanding host-microbiota immune and metabolic interactions that shape ISC function, such as the role of pattern-recognition receptors and microbial metabolites, including lactate and indole metabolites. Additionally, the diverse regulatory effects of the microbiota on dietary nutrients, including proteins, carbohydrates, vitamins, and minerals (e.g. iron and zinc), are thoroughly explored in relation to their impact on ISCs. Thus, we highlight the multifaceted mechanisms governing host-microbiota interactions in ISC homeostasis. Insights gained from this review provide strategies for the development of dietary or microbiota-based interventions to foster gut health.
Keywords: Intestinal stem cells; dietary nutrients; gut homeostasis; immune homeostasis; intestinal organoid; metabolic interaction; microbiome; micronutrients.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Mu C, Zhu W.. Understanding the relationship between the microbiome and the structure and function of the pig gastrointestinal tract. Understanding gut microbiomes as targets for improving pig gut health. 2022. doi:10.19103/AS.2021.0089.06. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
